Post-Viral Trials News
@postviraltrials
Followers
4,222
Following
163
Media
219
Statuses
3,922
News and updates about treatment trials for Long Covid, ME/CFS, POTS, and other post-viral and related illnesses. DM if participating in a trial or interested.
Joined March 2023
Don't wanna be here?
Send us removal request.
Explore trending content on Musk Viewer
Baekhyun
• 167149 Tweets
連続テレビ小説
• 81657 Tweets
Abema
• 54125 Tweets
BINI ON GLOBAL SPOTIFY
• 44967 Tweets
#ネプリーグ
• 43188 Tweets
#帰れマンデー
• 27494 Tweets
#despeinvi
• 27055 Tweets
VISIPrabowo MAJUKANbangsa
• 22326 Tweets
#アニマリング
• 21036 Tweets
ケンタッキー
• 19040 Tweets
サーティワン
• 18416 Tweets
全試合無料
• 17422 Tweets
BELIFT APOLOGISE TO NEWJEANS
• 17394 Tweets
篠澤広の肋骨
• 13648 Tweets
#ちいかわキラメキ
• 13327 Tweets
ウルリス
• 11996 Tweets
Last Seen Profiles
I urge anybody with any post-viral illness contemplating suicide or euthanasia to hold off making a decision for at least a year or two, until the big trial results are out. It’d be a shame to suffer for three (or worse, 30 years) years, only to miss treatment by mere months
I wonder if we need a Be Here for the Cure-style campaign, to help people weather the news cycle and the vagaries of the illness. Or just the simple message that it gets better, because it can (even if your physical circumstances don’t change).
#LongCovid
8
41
303
23
188
653
I have to break it up into two parts, but here’s the segment of German Chancellor Scholz answering a question from a Long Covid patient, with auto-translated auto-generated English captions. He says he personally knows a young man lying in a dark room all day…
Minute 47:10 -
@Bundeskanzler
antwortet auf die Frage zu Long Covid im heutigen Bürgerdialog in Erfurt
14
43
140
12
153
486
If you’re a non-Covid ME/CFS patient near NYC,
@VirusesImmunity
&
@PutrinoLab
are doing a study where they’ll give you FREE microclot and platelet activation imaging results! I apparently have both. Email PRcovid
@MountSinai
.org. They’ll come to your home (within 50 miles of NYC)
10
155
299
The German ME/CFS activists are running circles around the rest of us. The bed protest at the Bundestag, a banner in a packed stadium, the federal health minister showing up again and again to events – such an inspiration
6
46
284
Peluso, Deeks, Henrich & Hsue at UCSF (HIV background) just registered a monoclonal antibody trial for Long Covid (not yet recruiting). Based on previous statements/association with
@polybioRF
, seems like they are very interested in viral persistence
7
88
239
He keeps repeating how new it is, how we didn’t know “anything” about it, but over three years ago he himself said “you can see people who…have things that are highly suggestive of myalgic encephalomyelitis and chronic fatigue syndrome”
“Clinical trials likely should have been done sooner.”
On today's
@MehdiHasanShow
, I pressed Dr. Anthony Fauci on the slowness of our government to address what he calls the “serious problem” of Long COVID - and on Biden never mentioning it.
283
834
3K
11
38
214
Upsetting that the NIH got $1.15bn from Congress and their team of professional medical researchers, without any cognitive dysfunction, has not been able to find the same quality of grantees as LC patients themselves have with a tiny fraction of the financial/cognitive resources
This tremendously exciting paper is now published:
Exercise in
#LongCovid
induces severe tissue damage & skeletal muscle necrosis, metabolic disturbances, myopathy, & tissue infiltration of amyloid-containing deposits.
Patient-Led Research Collaborative helped fund this study!
46
1K
3K
4
45
185
I’ve tried so hard with this account to keep it very level-headed with researchers, but I can’t hold my tongue about what happened with RECOVER. Nobody at the NIH, Duke, NYU, or RTI leading this $1.15bn effort is qualified, and this “collegiality” may cost me a lot of my life
@postviraltrials
@DukeU
@duke
It’s really unfortunate to see you call my Duke colleagues “cruel”—including my colleagues at the IRB that approved these trials and my colleagues at NIH that funded these trials.
I had hoped that this might be a good faith discussion of science & data.
Muting this convo now.
0
0
2
7
28
173
Spoke with researchers yesterday (tons of experience with NIH and other grants) who said they’re looking for non-NIH options for LC/ME trial funding because NIH just takes too long (this one in
@DrJeanneM
’s world). Largest source of medical research funding has lost credibility
@coco_chatel
@SawyerBlatz
@MichaelPelusoMD
@PutrinoLab
@zalaly
Add Ron Davis to the list of critics. For one thing he’s been ready to go with a clinical trial of Abilify and can’t get it funded. There are many clinical trials that are ready to go but don’t have funding. That response is so disingenuous
7
34
207
8
39
171
The $1 billion/year Long Covid funding bill that
@SenSanders
introduced requires the NIH to set up a new grant process for clinical trials, “reviewed more quickly than traditional grants,” with priority for non-behavioral interventions
1
31
155
As I stew about the NIH, I’m looking through studies
@patientled
(partly) funded with just $4,800,000. Amazing how many not only were compelling ideas, but have ALREADY delivered results that moved the field forward not 1.5 years post-application deadline
6
22
138
Partial list of trials for Long Covid by
@Daltmann10
in Nature (missing some very important ones though – BC007, efgartigimod by Argenx, vericiguat by
@C_Scheibenbogen
, baricitinib by
@WesElyMD
, Paxlovid at Karolinska Institutet)
5
45
137
New Charité study: skeletal muscle biopsies from LC patients w/PEM show “fewer capillaries, thicker capillary basement membranes & increased numbers of CD169+ macrophages” than healthy controls, SARS-CoV-2 RNA undetected in muscle tissue. They hypothesize immune-mediated damage
Charité Studie: Hinweis für immun-vermittelte Schädigung der kleinen Gefäße der Muskeln bei
#PostCovid
Betroffenen mit Belastungsintoleranz:
36
234
683
2
39
130
Dr. Scheibenbogen’s group at the Charité in Berlin has published interim results of a small open label study of repeat immunoadsorption (depletes immunoglobulin levels through apheresis). Of 10 post-Covid ME/CFS patients with elevated ß2-adrenergic AAbs, 7 responded to treatment
5
29
127
If anybody who lives near the DC area is interested in the NIH’s IVIg trial for long COVID (fatigue, brain fog, orthostatic intolerance symptoms explicitly mentioned, among others), email angelique.gavin
@nih
.gov about recruitment. Trial details here:
11
62
126
I’m going to summarize the interview with Dr. Adrian Hernandez, at Duke, who is coordinating RECOVER’s trials. Dr. Hernandez’s comments are disappointing, and I think shed light on what went wrong with RECOVER
📣 Our podcast on Long Covid is now online
It begins with 30 mins of short interviews with Dr Fauci, Dr Iwasaki, patient advocate Fiona Lowenstein & others
Then we did a 30 min interview with the inspirational Dr Adrian Hernandez
@DukeU
Hope you enjoy
2
36
75
3
23
124
Interesting that they mention chronic fatigue syndrome even before MS and cancers in their list of sequelae. In Moderna’s investor material for its EBV vaccine in development, ME/CFS was never mentioned…times are changing
3
17
119
Peluso says to
@polybioRF
that his UCSF team is planning “a number of” small Long Covid trials of “immune system therapies or more complicated therapies” with lots of measurement in the next few months, more to understand the biology than to treat
4
31
119
Normally don’t focus on things so far off from a phase 2 trial, but this company’s cofounder is Klaus Wirth, who’s been active in this field for a while:
Both cofounders left Sanofi to start this firm, so they’ve got a lot of experience in pharma.
#mitodicure
launched its website:
We are the only
#MECFS
start-up company worldwide developing a novel targeted drug. Support innovative drug development aimed at healing ME/CFS, which is also the most severe form of
#LongCovid
.
#BMBF
#MECFSAwarenessDay
35
104
358
5
33
116
I think patients should channel out anger at this study towards a unified and consistent demand for the NIH to begin a study on the therapeutic option mentioned: immune checkpoint inhibitors. And demand that the NIH start it QUICKLY, without the delays of RECOVER
Prof.
@DrMaureenHanson
on the recent NIH intramural clinical study: “It's really imperative to start doing clinical trials for people who have been sick for decades.”
#MEcfs
#PwME
2
26
111
7
25
115
This is absolutely devastating. Beth was the only person with an ME/CFS diagnosis who I’ve ever met in person. Please call your reps (and if you knew her and where she lived, hers) and ask for funding for research in her memory.
3
7
114
A much more emphatic statement than I’ve ever seen from the NIH. Do they actually have the competence though, is the question…
“[
#LongCovid
] is a full NIH activity.. We must find better ways to.. restore the lives of these people”
“We are not where we want to be right now in terms of rapid, nimble clinical trials.. testing promising treatments.. that is our focus right now moving forward” —
@NIHDirector
16
98
337
13
14
113
FDA. If they put out a public statement saying they consider Long Covid to be an urgent matter and are open to issuing emergency use authorization for well-done phase 2 trials, I think we’d see more (and bigger) trials happening right now
Even though I’m a poli sci major & I’ve seen every episode of the West Wing — I found this to be very helpful lol
Besides HHS, Congress & WH — What other offices or departments have the power to expedite, fund or play a role in
#LongCovid
research ?
14
30
145
3
26
109
I think in his eagerness to dunk on Putrino and other clinicians and patients who believe post-viral ME/CFS is not just an elaborate form of hypochondria, Dr. Gaffney did not notice the entire study population was hospitalized, many in the ICU, and none showed signs of PEM/PESE
Notable development on the Long Covid front: consistent with previous studies, a randomized trial in
@bmj_latest
found that an exercise-based physical & mental health rehabilitation program improved outcomes for those with Long COVID.
44
31
100
7
17
108
Seven years from announcement to results. We cannot afford any more of these useless behavioral/psych intervention studies. No more money for Esther Crawley (), she’s had enough
1
17
106
The Bavarian government says that a trial of BC007 for non-Covid-onset ME/CFS is still planned by the University of Erlangen, and is negotiating with Berlin Cures about providing the drug
Wir haben eine Antwort vom Bayrischen Staatsministerium für Wissenschaft und Kunst erhalten, was eigentlich aus der Studie
#bc007
für
#MEcfs
(prä-pandemisch) werden soll. (Wir hatten auch dorthin geschrieben und nachgefragt)
#fundraisingbc00MEcfs
🧵
7
36
122
1
21
105
This is the problem with taking years to launch studies of already approved drugs that patients have already been experimenting with extensively – if patients aren’t already raving about it by then, it’s probably because it doesn’t work!
Stanford Paxlovid clinical trial was stopped early because it wasn't effective in treating
#longCOVID
26
116
372
8
16
105
The director of the National Heart, Lung, and Blood Institute is Gary Gibbons, and he likely has the power to fully fund Dr. Hwang’s team and trial. His office’s email is NHLBIinfo
@nhlbi
.nih.gov, note that it’s for Dr. Gibbons when you reach out
Excellent piece on
#MECFS
! It'll take a number of physicians and scientists like Dr. Hwang WITH FUNDING to solve
#MECFS
,
#Dysautonomia
and
#LongCovid
.
6
18
152
4
24
105
“PolyBio’s second phase of projects also includes funding for clinical trials to evaluate the impact of HIV antivirals on LongCOVID.” Will be directed by
@PutrinoLab
.
6
27
102
The NIH got 240 times as much money from Congress to study Long Covid (not including extra they’re taking from the general budget), and they haven’t delivered anything even half as compelling as just the
@RobWust
study alone. It makes me quite literally sick.
1
14
101
Hundreds of millions in RECOVER $$ went to studies like this – thousands of patients enrolled, and for what? To learn Long Covid involves PEM, fatigue & dizziness (so it’s ME & POTS), and anxiety, depression & poverty are LC risk factors. How does that help patients?
11
12
96
A very similar study looking to measure the same in patients with non-COVID ME/CFS is still recruiting! Requires one visit to Boston, see next tweet for details…
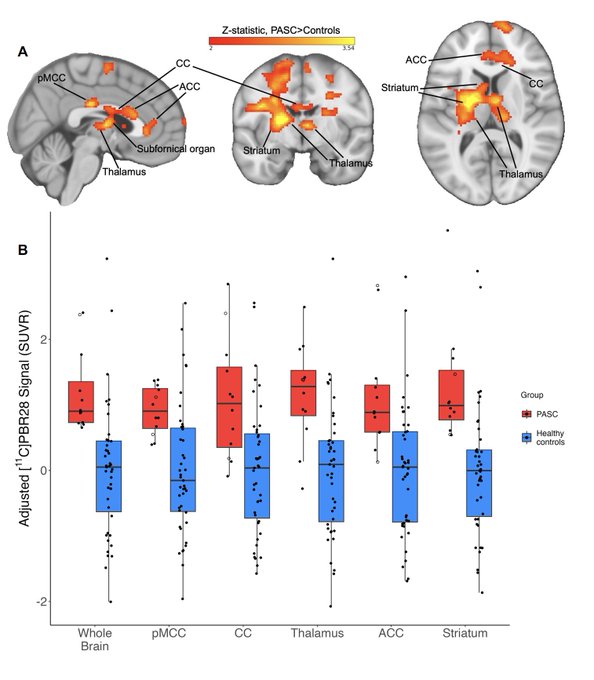
The diffuse inflammation of the brain in symptomatic people with
#LongCovid
by PET scans, with matched controls, and correlation with their blood inflammatory biomarkers
@MBVanElzakker
@MGHMartinos
@HannahFBues
0
523
1K
1
39
97
.
@PutrinoLab
says they’re finishing up with the last patients in the Mt. Sinai vagus nerve stimulation trial, and results should read out at the end of Q1 2024
1
16
97
Looks like we’re still on for June. Middle of this year should be big for Long Covid drug trials – we should have efgartigimod, temelimab, and I hope
@hopebio_org
’s stem cell results by then
5
21
94
@VirusesImmunity
@hmkyale
At 48:24 (last LC question), he mentions “chronic fatigue syndrome” as an example of condition that wasn’t taken seriously until the pandemic and still “hasn’t trickled down through healthcare.” Ironic given he hasn’t taken ME, POTS, etc. seriously at all designing RECOVER trials
2
7
94
From a
@PlzSolveCFS
presentation,
@PutrinoLab
discusses an unpublished finding from
@keylas3
and
@VirusesImmunity
: mice some exhibit balance and sensory issues (sounds like POTS and SFN?) after being injected with IgG from human Long Covid patients
5
29
94
David is underselling the benefits of participating – they give you microclot and platelet imaging and impressions! Here are mine:
A reminder that if you live within 50 miles of NYC and you have a PRE-2020
#MECFS
diagnosis, then
@VirusesImmunity
and I NEED your help! If you have difficulty traveling we will come to you. Please help us to hit our recruitment goals if you can! 🙏🙏
26
375
579
1
24
92
The NIH’s placebo-controlled trial of IVIg for Long Covid is still recruiting (contrary to the study page), you do NOT need to be invited. It’s three months of infusions in Bethesda, MD. Email study coordinator Ladi Fouanta at ladifatou.fouanta
@nih
.gov
8
41
88
Great presentation by
@hopebiosciences
with
@PlzSolveCFS
on their stem cell pilot study (no placebo control) in Long COVID. Five infusions of autologous cells (from their own fat tissue). Really good subjective results!
7
27
89
Beyond whole blood donations – what about plasma “donations” (which actually paid and would therefore be more likely to attract sick people, who perhaps cannot earn an income in other ways)? That’s where Ig product that goes into IVIg comes from. And now maybe it’s tainted? Yikes
@EricTopol
Why is the US still allowing patients with
#LongCovid
to donate blood? The UK doesn’t allow it. Not that we have the energy to donate anyways, but if it can happen to mice, I would feel terrible knowing that I gave another person my LC symptoms !
10
16
88
6
22
91
Bad news. After dogging the RECOVER press team, I found out that NIH’s RECOVER is double-counting the Paxlovid trial in their “13 interventions” claim, since they’re doing two dosing arms – one for 15 days, another for 15 days. So we’re down an “intervention” unfortunately
5
20
88
Japan’s National Center of Neurology and Psychiatry has IRB approval for a rituximab trial for ME/CFS, according to machine translation of this page (via
@MECFSNews
)
7
14
87
First patient dosed last month. Still recruiting in San Francisco (30 patients total, 2:1 blinding), email outsmartLC
@ucsf
.edu. Major criterion is that your Long Covid must have started before Aug. 15, 2022. I don’t see a PCR test requirement. More here:
Our first
@UCSF
#LongCOVID
clinical trial targeting viral persistence with monoclonal antibodies featured by
@NanetteAsimov
in the
@sfchronicle
today.
I am grateful to
@AeriumTx
@patientled
@polybioRF
for supporting this effort.
35
226
801
2
28
84
@VirusesImmunity
@hmkyale
“Suggest” vs. “note, well, actually” suggests to me that he doubts that PEM doesn’t improve with exercise. Beyond that, he seems to misunderstand that pacing is the practical application of the idea that PEM is not surmountable with gradual exercise, not an alternative hypothesis
2
9
84
@sebdave23
No Long Covid suicides allowed until at least 2025, too many trials ongoing right now. Sorry for the inconvenience but I don’t make the rules 🤷
1
2
81
Baricitinib trial for Long Covid is getting much larger
@PatientPersists
We’re converting this into a phase 3 trial which will enroll 550 patients. Awaiting final funding notice from NIH. More to come.
22
36
237
1
14
82
Berlin Cures appears to have added a Swiss trial site for BC007 phase 2 for Long Covid, in Zurich, at the city hospital. Contact is Lars Huber, +41 44 416 3001, lars.huber
@zuerich
.ch (speaks EN/DE/FR). Please, if you reach out, let me know how it goes!
2
23
80
First Long Covid patients at Floridsdorf in Austria expected to be dosed with BC007 in beginning of September, says
@lungendoc
. Preliminary results expected mid-2024, final results end 2024. Expects a phase III, but accelerated approval à la Paxlovid also possible, maybe by 2025?
Details zur
#BC007
-Studie für
#LongCovid
, die jetzt an Kliniken Floridsdorf und Favoriten von
@wiengesundheit
auch in 🇦🇹 begonnen hat.
Gut, dass endlich Bewegung in die Sache kommt und potentielle Therapien geprüft werden.
7
61
307
2
19
82
This one is enrolling finally! Let’s add a sixth to the Big Five trials. Looks like it’s by invitation only. If anybody knows anything more about it, or is asked to enroll (or reaches out themselves), my DMs are open
UCSF, led by
@MichaelPelusoMD
, is starting this trial with the anti -SARS-CoV-2 spike antibody cocktail AER002.
Why I'm so excited that this can cure viral persistence in Long Covid:
9
74
238
3
20
80
Triemli Hospital (Zurich) says they’ll enroll for BC007 in fall/end of 2023. Must have gotten Long Covid from an infection no more than 12 mos. ago, need a PCR or “official antigen test.” Send email with test result to studielongcovid
@stadtspital
.ch
4
23
78
That wasn’t the finding at all. This wasn’t a trial, there was no intervention. It’s simply noting a correlation between symptoms and physical activity.
Exercise reduces long-term COVID symptoms in young women 🏋️♀️🧠❤️
#ExerciseHeals
#COVID19Recovery
#YoungWomenFitness
#PostCOVID
#MentalHealth
#Neurological
#Health
#StayActive
#COVID
#Health
#PhysicalActivity
@SciReports
69
4
10
2
12
81
So many well understood autoimmune disorders have unique manifestations apparent to outside observers (drooping eye in myasthenia gravis, rash in lupus, sweet urine in T1D). How many others are we missing because they present “only” as nondescript fatigue, pain, or psychosis?
I stand by my mantra. Every disease should be considered immunological, until proven otherwise.
#neuroimmunology
18
68
265
4
14
78
At a conference today,
@argenxglobal
defined what it believes is the size of the US post-Covid POTS market: 500,000 patients (330,000 for Sjögren’s). Both phase 3 trials could start in 2024 (contingent on good phase 2 results, due first half of this year)
11
19
79
PLEASE do this! One or two sentences. Goal is not to get them to publish the letters, but to have editors hear interest. These stories get read at the highest levels at the NIH, but the Times won’t keep writing them if they don’t get any feedback, because they get little traffic
I used to be a reporter in a past life. At the Times especially, editors do not let columnists and reporters write about just whatever, especially not kinda low-traffic things like this. If you want to see more Long Covid coverage, email them and tell them! letters
@nytimes
.com
6
21
77
5
28
78
I used to be a reporter in a past life. At the Times especially, editors do not let columnists and reporters write about just whatever, especially not kinda low-traffic things like this. If you want to see more Long Covid coverage, email them and tell them! letters
@nytimes
.com
6
21
77
Given that ME/CFS (Covid-onset or not) is fundamentally an activity-limiting disease, it would make a lot of sense for trials to use number of daily steps as an endpoint
4
3
75
A doctor involved in an open label study of immunoadsorption for patients with Long COVID with ß2 autoantibodies says the “vast majority” of patients respond to the treatment, but then they deteriorate after three months, as the autoantibodies return
5
16
75
Spot on column about Long Covid and the US government. So much in there, but I especially appreciated the recognition of
@meighanstone
’s not only work in Washington, but also financial contribution to bring patients there in person
4
22
76
@NateB_Panic
@billsonofbill2
Nate, that is absolutely not true. POTS was a common post-viral condition even before Covid. Many common circulating viruses cause it besides SARS-CoV-2. If you have no reason to believe she had Covid this summer, please respect her life and delete this misinformation.
1
2
76