Maciej Meglicki
@mmeglicki
Followers
38
Following
159
Media
0
Statuses
26
I just spotted this post and it is nearly 5 years later and the new answers brought new questions to track … if your interested in early development and symmetry breaking and self-organization, please get in touch with us, ideally by email as our lab don’t check X regularly
We are looking for postdoctoral colleague to study cell fate choices using lights sheet and 2photon imaging of mouse natural and stem cell-derived embryos in California @Caltech. If you are interested, pls send me a message. Please RT.
0
3
50
Amazing opportunity to learn more about embryogenesis, the placenta and fetal growth! Spread the word and we are looking forward to seeing you in Cambridge this July!
#announcement please share 📢 Annual Meeting 2025 'The Placenta at Term' registration live https://t.co/xfvUnO2R2Y Applications open for Placental Biology Course, in-person, in Cambridge this September https://t.co/CiGfKKF5lk 25 places, application deadline 21 March!
0
4
12
Curious embryo fact 9/25 Primordial germ cells (PGCs) give rise to eggs in females and sperm in males. In the 4th week, primordial germ cells leave the embryo! They migrate into the yolk sac. Thus, the next generation – the precursors to the offspring of the developing embryo
1
9
63
Women lose >90% of their #eggs by their mid-30s, with persistent #DNADamage driving this loss. But why isn’t this damage repaired? Our new work in @CurrentBiology, led by @ninadini_sharma, reveals the causes of high DNA damage in aged oocytes.🧬💥 https://t.co/hd8iT1TKnJ (1/13)
13
256
1K
BREAKING NEWS The 2024 #NobelPrize in Physiology or Medicine has been awarded to Victor Ambros and Gary Ruvkun for the discovery of microRNA and its role in post-transcriptional gene regulation.
545
12K
28K
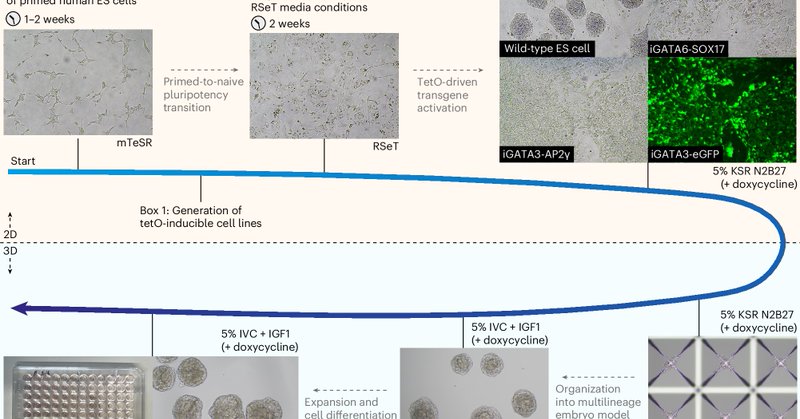
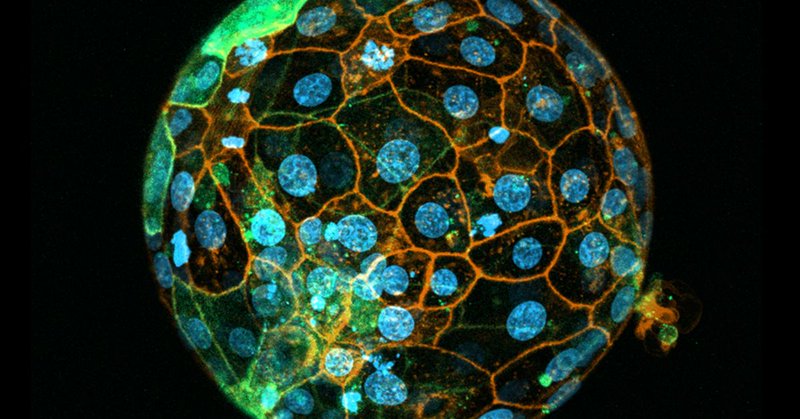
After reporting our embryoid model last year, @_carlosgantner_ and I wanted to put a protocol together. Out today in @NatureProtocols: https://t.co/zNtAM7S8fN Big thanks to Carlos for spearheading, Yuntao Wang for the help, and @ZernickaGoetz/@MZG_Lab for supervising!
nature.com
Nature Protocols - Protocol for the generation of a stem cell-derived human postimplantation embryo model by the combination of embryonic and transgene-induced extraembryonic-like cells.
Pleased to share our work in @Nature. https://t.co/DxQIBVSpBE I co-led this project with @_carlosgantner_ and we are so grateful to our co-authors (@LIwamotoStohl, Riza Daza, @Nobu_Hamazaki, @JShendure, & @ZernickaGoetz), editors, and reviewers. 1/
0
3
28
I’ve been presenting our human stem cell embryo model, derived from programmed human ESCs, at conferences for a few years and last year, we finally published it. Now we’re excited to share a detailed protocol how to create these models #StemCellResearch
https://t.co/JqipYyEQco
nature.com
Nature Protocols - Protocol for the generation of a stem cell-derived human postimplantation embryo model by the combination of embryonic and transgene-induced extraembryonic-like cells.
3
14
106
It's that time of year!! 🎉We are so excited to announce our 2023 pick for Nature Methods' Method of the Year: methods for modeling development. Read the Editorial to find out why we chose this as our pick. #moty2023
https://t.co/RNLeOZLE52
12
217
626
Excited to share our preprint! We delve into the ways that a mouse embryo breaks symmetry and specifies cell fates for the first time. It turns out that cells polarize asynchronously at the 8-cell stage, and this has implications for lineage allocation: https://t.co/jHtbnulr87
biorxiv.org
The first lineage allocation in mouse and human embryos separates the inner cell mass (ICM) from the outer trophectoderm (TE). This symmetry breaking event is executed through polarization of cells...
4
12
58
“I was taken by the beauty of the complexity,” @ZernickaGoetz said about the embryos she’s studied for almost three decades. Read about her research and leadership in this Peer Profile:
the-scientist.com
Magdalena Zernicka-Goetz followed the aesthetic allure of the embryo to better understand the start of development.
0
7
34
Women are born with all their oocytes, which need to stay viable for decades to ensure fertility. How are they maintained for that long? Our latest research in @NatureCellBio reveals that oocyte maintenance involves exceptional protein longevity. https://t.co/ZiByc9F4hS (1/9)
18
285
1K
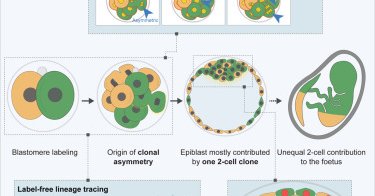
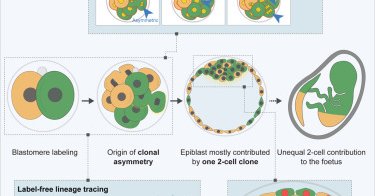
The @MZG_Lab is back at it again with a new @CellCellPress paper! They used lineage tracing to show that the majority of the #epiblast is derived from only one 2-cell stage blastomere. @JunyentSergi @mmeglicki 🔖 https://t.co/GdFRgTWnU4 🎧 https://t.co/dL7CAhoLmm
0
3
31
I'm very excited to share another paper from our @MZG_Lab ! Great work by amazing Meng Zhu (@zhumeng123) and everyone else participating! I'm proud I could contribute to this work as well :)
Excited to share our new work!!🥳How do cells lose totipotency to fate segregation? w @MZG_Lab we found that early blastomeres experience a "Composite State" by displaying dual-lineages signatures! Link for full text👉: https://t.co/n10KchKhda
1
1
9
Now online! The first two blastomeres contribute unequally to the human embryo
cell.com
Labeling and live imaging of human embryos reveal that the majority of the future body originates, mostly, from one of the 2-cell stage blastomeres. Descendants of the first 2-cell stage blastomere...
0
42
188
I’m very excited to share that our paper “The first two blastomeres contribute unequally to the human embryo” has been published today in Cell. Many thanks to everyone collaborating in this project, especially to @ZernickaGoetz (Magdalena Zernicka-Goetz) and @JunyentSergi!
What is the origin of our body? Our paper @CellCellPress shows that when a human embryo is one day old and comprises just two cells, only one cell will create most of the fetus in addition to placenta, while the other cell will create placenta. https://t.co/88l48YezzA
0
1
7
What is the origin of our body? Our paper @CellCellPress shows that when a human embryo is one day old and comprises just two cells, only one cell will create most of the fetus in addition to placenta, while the other cell will create placenta. https://t.co/88l48YezzA
cell.com
Labeling and live imaging of human embryos reveal that the majority of the future body originates, mostly, from one of the 2-cell stage blastomeres. Descendants of the first 2-cell stage blastomere...
34
340
1K
Amazing cover and amazing work from our lab! Congratulations Kasey, @GianlucaAmadei1 and @_carlosgantner_ ! It's been a pleasure to see this story in the making!
Our October issue is live! Highlights include:a post-gastrulation all ESC-derived mouse embryo model, advances in template delivery for HSC gene editing, iPSC arrays for GWAS discovery, vaccine-based immunotherapy against fibrosis & reprogramming synergies https://t.co/aGkqvAoDP3
0
0
6