Raghu Chivukula, MD PhD 🫁🧬🧠
@chivukula_raghu
Followers
892
Following
7K
Media
18
Statuses
941
physician-scientist @harvardmed @MassGeneralNews & @CGM_MGH | dadx2 | porschephile | via @JohnsHopkins @HopkinsMedicine @MGHMedicine @HarvardPulm @WhiteheadInst
Boston & Camberville, MA
Joined January 2020
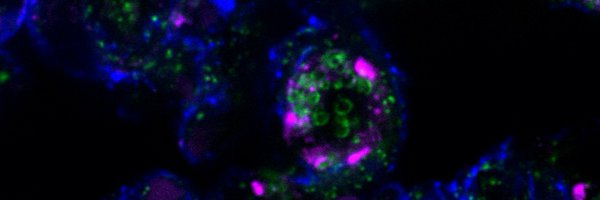
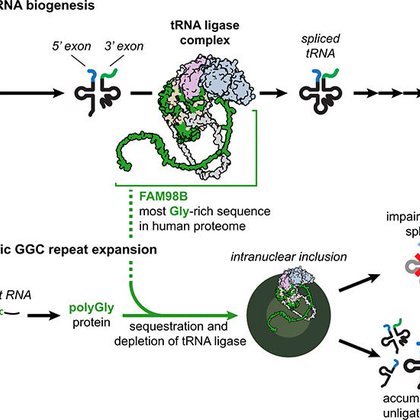
Why do some genetic mutations devastate specific tissues while sparing others?. We explore this question in GGC repeat disorders, revealing a mechanism linking protein aggregation to tRNA splicing failure in the brain. 🧵 on our new paper in Science:.🔗
science.org
Aggregation-prone polyglycine-containing proteins produced from expanded GGC repeats are implicated in an emerging family of neurodegenerative disorders. In this study, we showed that polyglycine...
14
52
191
RT @DKThomp: One lesson of DOGE is that a lot of federal govt spending is sent to old ppl (social security, Medicare) and affluent individu….
0
657
0
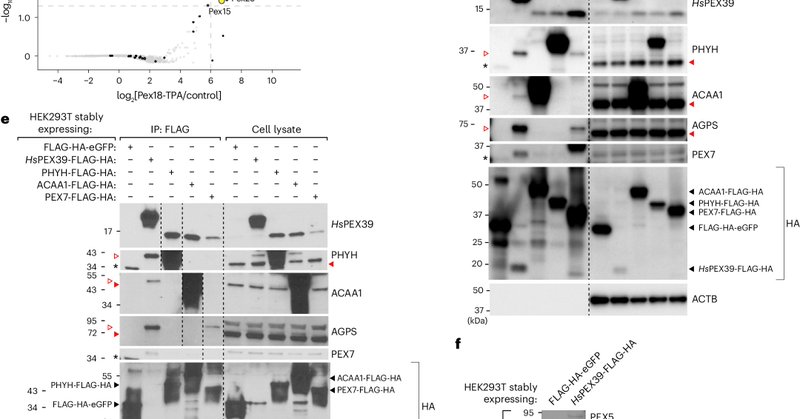
RT @WalterWChen1: Our paper on discovering a new paradigm in peroxisome biogenesis and PEX39 (1st human peroxisomal biogenesis protein fou….
nature.com
Nature Cell Biology - Chen et al. show that PEX39 cooperates with PEX7 in the peroxisomal import of proteins containing a PTS2 site and uncover an (R/K)PWE motif in PEX39 and PEX13 that binds to...
0
27
0
RT @YH_YukiBio: Excited to share our new preprint on @biorxivpreprint from our great team @EMBL!. If the nucleolus….
0
32
0
RT @rbarbosa91: Practicing knot tying is underemphasized in the training of those in non-surgical specialties. Many years of observation h….
0
35
0
RT @LukeKoblan: Excited to announce that this work is now live as a first release article @ScienceMagazine So grateful for this fantatic te….
science.org
Charting the spatiotemporal dynamics of cell fate determination in development and disease is a long-standing objective in biology. Here we present the design, development, and extensive validation...
0
19
0
RT @ImranSHaque: I find trinucleotide repeat disorders fascinating; between this new paper from @chivukula_raghu on GGC repeats and one ear….
0
2
0
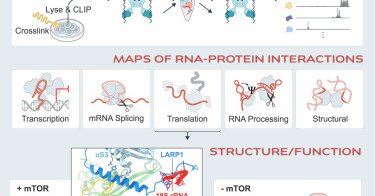
RT @CellCellPress: Now online! SPIDR enables multiplexed mapping of RNA-protein interactions and uncovers a mechanism for selective transl….
cell.com
SPIDR, a massively multiplexed method that simultaneously maps dozens of RNA-binding proteins to their RNA targets at single-nucleotide resolution, uncovers new RNA-protein interactions and provides...
0
18
0
Finally, we are grateful to funders including @BWFUND,.@ChenInstitute, Smith Family Foundation,.@MassGenBrigham, @MGHMedicine, @MGHSurgery, @CGM_MGH for supporting our work!. Thanks for reading!.🔗
science.org
Aggregation-prone polyglycine-containing proteins produced from expanded GGC repeats are implicated in an emerging family of neurodegenerative disorders. In this study, we showed that polyglycine...
1
0
4
This work was led by @jason_yang0 (now @BrunetLab) and aided by a talented and hardworking team: Forrest Xu, David Ziehr, @DrMartyTaylor @mlvalenstein @genfren Jack Bush, Kate Rutter, Igor Stevanovski, @CharlieYShi Mahesh Kesavan, @RMouroPinto @IDeveson David Bartel @DMSabatini.
1
0
6
We believe selective vulnerability reflects cell type–specific demands on molecular systems like RNA processing, translation, proteostasis, and secretion, among others. Want to learn more?. 🚨 Postdoc openings now available.📍 MGH / HMS / Broad.📩 rchivukula@mgh.harvard.edu.
1
0
1