Amir Giladi
@AmirGiladi
Followers
880
Following
2K
Media
10
Statuses
244
Studying immune function and immune interactions with single cell genomics. Post-doc at the Hubrecht institute in Van Oudenaarden and Clevers labs.
Joined November 2017
Amazing work from the wizard @DanielKruegerDK and co.
1/4 Gut renewal is not passive “crowd control.” Instead of being pushed out by crowding or dying from apoptosis, cells compete in a mechanical tug-of-war. Weaker cells are eliminated—reframing intestinal homeostasis as a force-regulated process.
1
0
2
Presenting our most popular post of 2024! 🥂 📯 Awarded to Lulu Huang, Dr. Jochem Bernink, Dr. @AmirGiladi, Dr. @HansClevers, and the team at @_Hubrecht. Their study identifies distinct tuft cells in the human intestine. https://t.co/ZVjHvv9ZWi
Check out the latest release from Dr. @HansClevers' lab! 🧫 Lulu Huang, Drs. Jochem Bernink, @AmirGiladi, and their @_Hubrecht team reported #TuftCells as a damage-induced reserve intestinal stem cell pool in humans. 📖 Get the full story in @Nature. https://t.co/YMyWni8mW9
0
1
12
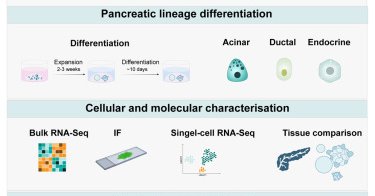
Exciting study by Amanda Andersson Rolf et al: In vitro expansion of a human fetal #pancreas #stemcell that generates all three pancreatic cell lineages”. https://t.co/mk4MdczkTM 1/
cell.com
Identification of an LGR5 as a marker for a tripotent stem/progenitor cell of the human fetal pancreas. Organoids derived from single LGR5+ cells are capable of long-term expansion in vitro and...
2
32
165
A new @Nature study from Lulu Huang, Dr. Jochem Bernink, Dr. @AmirGiladi, and a team in Dr. @HansClevers' lab explores how #TuftCells in the intestine regenerate after irradiation! ☢️ Check it out: https://t.co/5z70wIsDYf Listen to the summary: https://t.co/wArKMQWGOZ
0
1
6
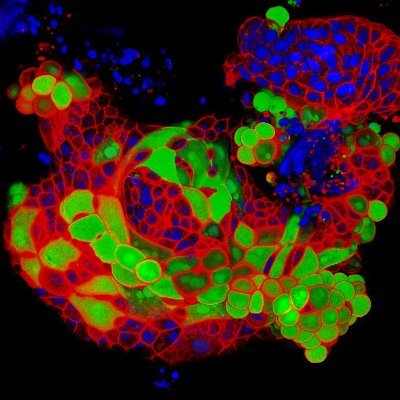
Thank you @BPoD_s for highlighting this beautiful tuft cell (image taken by Lulu Huang and @DanielKruegerDK).
Tuft cells act as stem cells able to regenerate the human intestinal lining after damage 📷: Lulu Huang, Jochem H. Bernink & @AmirGiladi et al @_Hubrecht in @Nature ➡️: https://t.co/iLhRFfr6BY
0
2
5
This has been a collaborative effort led by Lulu Huang, Jochem Bernink, and myself from the organoid group of @HansClevers. We thank our collaborators from the organoid group, the AUMC and PMC for their invaluable contributions. Happy new year :)
0
0
3
We thus suggest a model where upon loss of the LGR5+ stem-cell pool, IL-4 wound-healing signaling can prompt tuft cells to proliferate and trans-differentiate, marking them as the long sought-after damage-induced reserve stem cell in the human intestine.
1
0
2
To fully understand tuft-cell stemness capacity, we irradiated tuft cell lineage tracing organoids. Strikingly, we find that in this damage-inducing setting, tuft cells not only survive irradiation, but can reconstitute entire epithelial organoids.
1
0
1
Using an AVIL lineage-tracing reporter, we found that a single proliferating tuft cell has the capacity to form organoids that contain all epithelial lineages. After passaging, these organoids become indistinguishable from LGR5+ stem cell-derived organoids.
1
0
1
We mined for transcription factors associated with tuft cell identity. A screen of TF-knockout organoids revealed a high dependency on the Wnt-target TCF7 and POU2F3. HMX2 didn’t affect tuft cells numbers in naïve organoids, but blocked tuft cell expansion under IL-4 treatment.
1
1
2
scRNA-seq of naïve and IL-4-treated organoids identified 4 tuft cell states. IL-4 signaling dramatically shifts tuft cell composition toward cells entering the cell cycle, and the rest up-regulating a transcriptional program associated with activation of the immune system.
1
0
1
The ability of mature human tuft cells to enter the cell cycle and proliferate was further validated both in vitro and in primary intestine tissue.
1
0
1
In mice, IL-4 signaling from innate lymphocytes promotes stem cell differentiation toward tuft cells. We observed a ten-fold increase in tuft cells in IL-4 treated human organoids. Surprisingly, this expansion was due to self-propagation from pre-exsiting cycling tuft cells.
1
0
2
We used AVIL reporter organoids to optimize permissive culture conditions for tuft cells. We discovered that tuft cell formation is highly dependent on Wnt activity. In line with this finding, tuft cells in the human intestine tissue localize favorably to the Wnt-rich crypt area.
1
0
1
We genetically engineered organoid models to study human tuft cells. We mined open atlases of human primary tissue and identified Advilin (AVIL) as a broad yet specific tuft cell marker. AVIL+ tuft cells in organoids display the unique brush-like morphology as observed in vivo.
1
0
1
Our knowledge on tuft cell biology comes mostly from mouse models, where they were shown to interact with immune cells to facilitate anti-microbial defense. Lack of genetic tools to investigate human tuft cells impeded our ability to study their role in the human intestine.
1
0
1
We are excited to share our new research article, in which we, for the first time, identified a human intestinal reserve stem cell, namely tuft cells, by combining novel organoid models and single-cell genomics (link: https://t.co/ZN5mqLMFWL).
10
9
73
Psychologist Daniel Kahneman (1934–2024) "changed how people thought about decision-making" through his work with the late psychologist Amos Tversky. Read more about his life in a new #ScienceRetrospective: https://t.co/y74clpFirW
3
40
168
What happens when cells stop assemblying nucleosomes during DNA replication? We used CAF-1-degron human cells to answer this question, and look at the immediate effects within a single cell cycle. 🧵⬇️ https://t.co/iZO5GHxc2N
biorxiv.org
Long-term perturbation of de novo chromatin assembly during DNA replication has profound effects on epigenome maintenance and cell fate. The early mechanistic origin of these defects is unknown....
1
24
69