Yongliang Zhang
@zhang_yongliang
Followers
139
Following
379
Media
1
Statuses
52
Professor @ Xi’an Jiaotong University
Xi'an, China
Joined November 2019
RT @LevinChem: Today in @ScienceMagazine, we report a method to replace the C2 of pyridines with N, affording pyridazines. The change fr….
0
90
0
RT @LevinChem: How do you turn a deletion into an insertion? In our latest, Myojeong shows that the anomeric amide can N-delete *and* re-a….
0
53
0
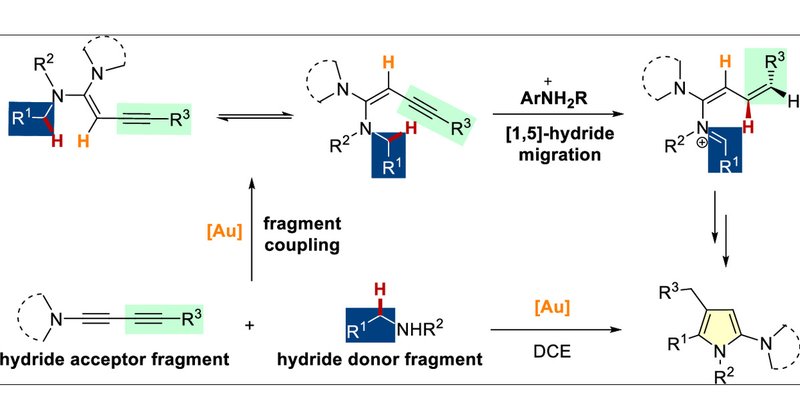
Our research on gold-catalyzed intermolecular assembly was published in Organic Letters. The first research project I participated in @XJTU.
pubs.acs.org
The [1,n]-hydride migration reactions represent one of the most powerful techniques to functionalize C(sp3)–H bonds and to rapidly construct molecular complexities. However, their substrates usually...
0
0
5
RT @InorgChemFront: 🔥 Don't miss this #HOT article by Wen-Xiong Zhang et al. @PKU1898:. "sp2–sp cross-carbanion coupling at a rare-earth ce….
0
2
0
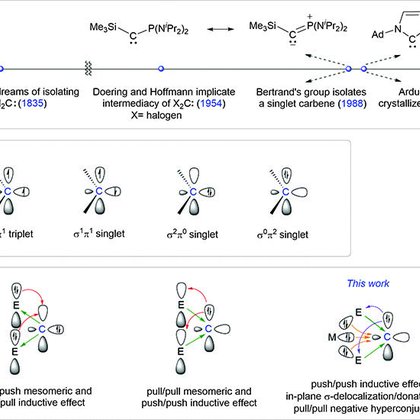
RT @LLL_lab_SUSTech: Happy New Year 2024. Our latest research on a stable singlet carbene with a reversed electronic configuration is now p….
science.org
A divalent carbon atom hosts two electrons in an orbital perpendicular to the plane of its bonds to adjacent atoms.
0
48
0
RT @CCSChemistry: Highly Stereoselective Synthesis of 2-Azido-2-Deoxyglycosides via Gold-Catalyzed SN2 Glycosylation .
0
12
0
Dinitrogen Activation by Titanium Hydride Complex Supported by 2-Butene Ligand - now published in Inorganic Chemistry Frontiers
pubs.rsc.org
The activation of dinitrogen by a titanium hydride complex with two bridging hydrides located between metal Ti and Li was investigated. Exposing cyclic bis-alkylidene titanium complex 1 to H2 in THF...
1
0
7
RT @LevinChem: Today we report a new strategy for C-to-N atom replacement in heterocycles. Led by @ChemieJisoo, with Colin Stein (visiting….
0
146
0
RT @angew_chem: How do Jiaxiang Chu and Dominik Munz’s friendship and postdoc collaboration continue to reap benefits for their research gr….
0
5
0
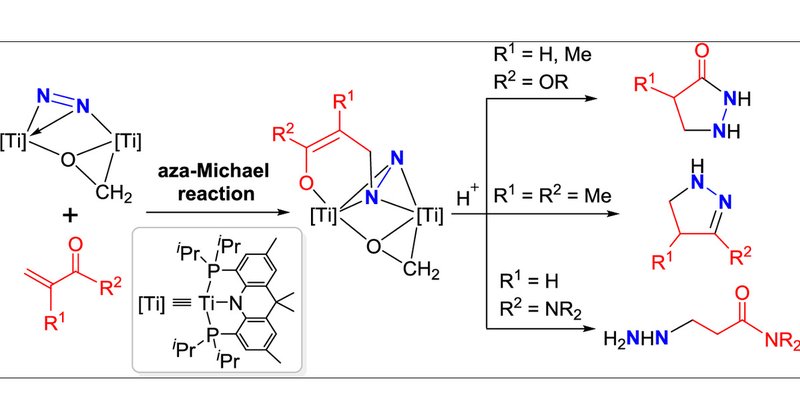
RT @J_A_C_S: Aza-Michael Addition of Dinitrogen to α,β-Unsaturated Carbonyl Compounds in a Dititanium Framework | Journal of the American C….
pubs.acs.org
The direct use of dinitrogen (N2) as a building block for the synthesis of NN-containing organic compounds is of fundamental interest and practical importance but has remained a formidable challenge...
0
7
0
RT @LevinChem: The final version of this work is out now in Organic Letters @JOC_OL . New since May - explicit demonstration of [11C]CO2 re….
0
20
0
RT @ChineseChemSoc: .@CCSChemistry Article Highlight: One-electron reduction of biferric nitrogen complexes under spatial constraints . Cli….
0
8
0
Here is our recent work: Highly Stereoselective Synthesis of 2-Azido-2-Deoxyglycosides via Gold-Catalyzed SN2 Glycosylation | CCS Chemistry is the flagship journal of the Chinese Chemical Society.
chinesechemsoc.org
Highly stereoselective synthesis of 2-azido-2-deoxyglucosides and 2-azido-2-deoxygalactosides is achieved via a gold-catalyzed SN2 glycosylation. The glycosyl donors feature a designed 1-naphthoate...
5
0
13
RT @group_levin: Interested in skeletal editing? Come check out our undergrads at #ACSFall2023!!
0
6
0
In this paper, the challenging enantioselective dearomatization of phenols was achieved by us. Big thanks to Liming, Ke, Xinyi, and Carlos~.
Asymmetric Dearomatization of Phenols via Ligand-Enabled Cooperative Gold Catalysis (Liming Zhang and co-workers) @ucsbchemistry
2
1
8
RT @yang2biocat: We published our first paper on pyridoxal radical biocatalysis in @ScienceMagazine : What we are….
0
77
0
RT @yang2biocat: Our paper on aromatic radical cyclases is out in @NatureCatalysis: We expanded metalloredox radica….
0
16
0
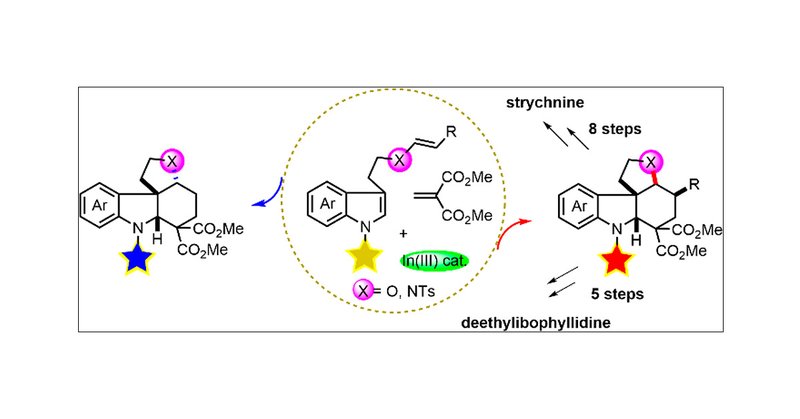
RT @J_A_C_S: Insights into Stereoselectivity Switch in Michael Addition-Initiated Tandem Mannich Cyclizations and Their Extension from Enam….
pubs.acs.org
Both cis- and trans- tetracyclic spiroindolines are the core of many important biologically active indole alkaloids, but the divergent synthesis of these important motifs is largely hampered by the...
0
9
0
Check out our latest paper for a new and general approach to SN2 glycosylation by employing an amide-directing group.
Directed SN2 Glycosylation Employing an Amide-Functionalized 1-Naphthoate Platform Featuring a Selectivity-Safeguarding Mechanism @ucsantabarbara @TotalSyntheses #SN2 #Glycosylation #Naphthoate #Saefeguarding #Mechanism
1
1
5