Ravi Uppaluri, MDPhD
@DrUppaluri
Followers
1K
Following
1K
Media
48
Statuses
323
Director, Head and Neck Surgical Oncology, Chief of Otolaryngology, Brigham and Women’s and Dana-Farber Cancer Institute
Boston, MA
Joined March 2016
Had a lot of fun discussing perioperative trials in LA-HNSCC with @deepmargin_pod @will_oncology BenO'Leary !!! KEYNOTE-689, NIVOPOSTOP and future directions! @DrHaddadRobert @imrtlee @HeadNeckMD
https://t.co/VAVZpldgpl At end we discuss @jvuppaluri favorite @premierleague team!
open.spotify.com
Deep Margin · Episode
0
3
14
Could perioperative immunotherapy change head and neck cancer care? On #ENT245, Drs. Luginbuhl, Roden, and Uppaluri unpack the Keynote-689 trial and use of #pembrolizumab in locally advanced disease.@_backtableONC @DrUppaluri @HemeOncBuddy @safaviaa
https://t.co/l0yux1ljDe
open.spotify.com
BackTable ENT · Episode
0
3
8
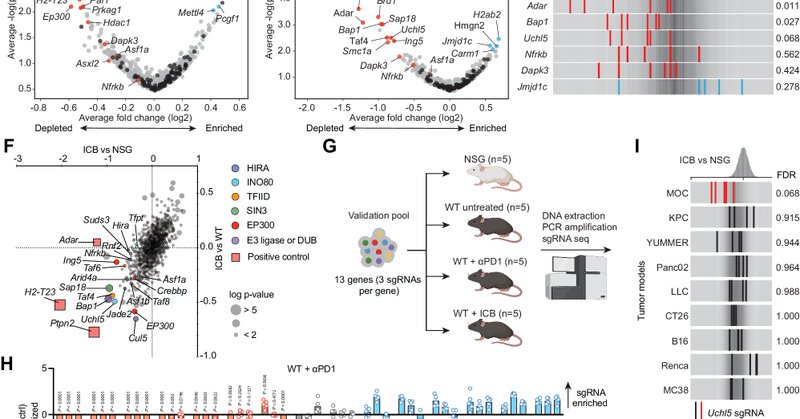
Our latest work on IO target discovery is out this week. Amazing collaboration with @DrUppaluri and led by Cong Fu. We performed in vivo CRISPR screens in #HNSCC to identify targets to enhance ICB response. @MGHCancerCenter @broadinstitute @DanaFarber
https://t.co/pikSB4I2SE
nature.com
Nature Communications - UCHL5 is a deubiquitinating enzyme that cleaves Lys-48-linked polyubiquitin chains. Here, the authors discover through in-vivo CRISPR-Cas9 screens that Uchl5 is involved in...
2
7
32
Congrats to DrCongFu ! Wonderful to collaborate with @MangusoLab on in vivo CRISPR of epigenetic targets study in #HNSCC models @NatureComms @kathleenbyates @IOTNmoonshot Thanks also to @SilvioGutkind @rsaddawi !!
nature.com
Nature Communications - UCHL5 is a deubiquitinating enzyme that cleaves Lys-48-linked polyubiquitin chains. Here, the authors discover through in-vivo CRISPR-Cas9 screens that Uchl5 is involved in...
0
11
38
Interim results from KEYNOTE-689 (NEJM): Perioperative pembrolizumab + standard care improved 3-yr EFS in locally advanced HNSCC (CPS ≥10: 59.8% vs 45.9%). Led by @DrUppaluri. Details: https://t.co/OFNzwEz7mI
#KEYNOTE689 #HNSCC #Oncology
1
1
25
Tomorrow is the #SITC Summit on the Future of Neoadjuvant Clinical Trial Design, which will discuss the recent advances in clinical trials for resectable tumors. The Neoadjuvant Clinical Trial Design Working Group is chaired by Patrick Forde, MD. Register: https://t.co/m9VDVO2Q23
2
16
47
Here it is! KEYNOTE-689 primary publication online now @NEJM. It’s been an incredible journey with DAdkins @SitemanCenter @WUSM. All started in early 2010s / now with @US_FDA approval and a new standard of care! @DanaFarberNews @BrighamWomens @BrighamSurgery
5
31
89
FDA approves pembrolizumab for resectable, locally advanced HNSCC based on KEYNOTE‑689. Led by Dr. Ravindra Uppaluri @DrUppaluri, the study showed a 30% drop in recurrence risk. Full details from The ASCO Post: https://t.co/ZLzVPI9Jn5
#HNSCC #Oncology
0
8
22
Exciting news in the head and neck cancer space. KEYNOTE-689 resulted in the first FDA approval for patients with HNSCC in 6 years. Congrats to @DrUppaluri, Doug Adkins, and the rest of the team worldwide. https://t.co/DftVyMSk2C
fda.gov
On June 12, 2025, the Food and Drug Administration approved pembrolizumab (Keytruda, Merck) for adults with resectable locally advanced head and neck squamous c
0
5
19
The results of the KEYNOTE-689 trial that served as the basis for this approval were recently presented by @DrUppaluri of @HarvardMed and @BrighamWomens at #AACR25. Read more about the trial results on the #AACRBlog:
aacr.org
Adding pembrolizumab before and after surgery to standard of care improved outcomes in previously untreated head and neck cancer patients.
0
4
6
#BREAKING The @FDA has approved pembrolizumab as the first new standard-of-care treatment for patients with resectable locally advanced #HeadandNeck cancer in 20+ years. Approval based on the pivotal KEYNOTE-689 trial led by @DrUppaluri of the @DanaFarber Brigham Cancer Center.
2
14
77
Today’s #FDA approval marks a significant step forward for certain patients with head and neck squamous cell carcinoma. Learn more here: https://t.co/p6MHJAbgQ6
3
16
35
Thanks @DrGopalIyer !!! Has been quite a journey from an idea we had in the early 2010s to change standard of care (with DAdkins at WashU as a partner on all this work)- exciting news for our patients.
@DrUppaluri Not everyday someone gets to change practice.. this is why we all do what we do….this is the dream!!!!
0
0
10
The standard of care has finally changed for patients with resectable head and neck #cancer!! Congratulations @DrUppaluri and all the #KN-689 investigators. https://t.co/DE3U7kBH3m
fda.gov
On June 12, 2025, the Food and Drug Administration approved pembrolizumab (Keytruda, Merck) for adults with resectable locally advanced head and neck squamous c
0
13
27
Boom!💥 @FDA approves neoadjuvant and adjuvant pembrolizumab for resectable locally advanced head and neck squamous cell carcinoma, ushering in a new standard of care for the first time in decades.
fda.gov
On June 12, 2025, the Food and Drug Administration approved pembrolizumab (Keytruda, Merck) for adults with resectable locally advanced head and neck squamous c
0
21
37
If you are attending @AHNSinfo, please join Dr. Stephen Lai & me this evening (Wed) in Celestin E at 6:15pm for updates on @NRGonc HN006 AND to hear PRACTICE CHANGING data from Keynote 689, the Ph3 trial of perioperative pembrolizumab recently presented @AACR by @DrUppaluri
0
2
11
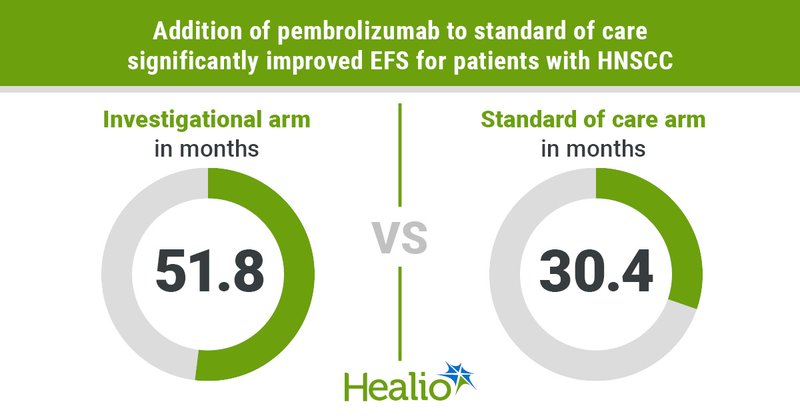
“It’s really exciting. It’s a new frontier for our patients.” @DrUppaluri of @DanaFarber discussed data from KEYNOTE-689 trial that showed @KEYTRUDA significantly extended EFS for patients with advanced HNSCC. @AACR @BrighamWomens @harvardmed
https://t.co/8hG5OrrZ9d
healio.com
The addition of neoadjuvant and adjuvant pembrolizumab to standard of care significantly improved outcomes for certain patients with advanced head and neck squamous cell carcinoma, according to...
0
4
8