Canac Yves
@CanacYves
Followers
428
Following
4K
Media
0
Statuses
938
CNRS Research Director: Main-group Chemistry, Synthetic chemistry, Organometallic chemistry, Coordination chemistry, Homogeneous catalysis.
LCC_CNRS Toulouse, France
Joined September 2019
Our review on "Molecular Design and Redox Chemistries for Aqueous Organic Redox Flow Batteries (AORFBs)" is now online as an open-access paper! 🥂 Please feel free to check it out! https://t.co/jxDDOWhnlY
2
9
45
Our lastest article has just been released (Org. Biomol. Chem): Synthesis, duplex-forming properties, and enzymatic resistance of oligonucleotides containing N-acylsulfonamide linkages https://t.co/e4yMN25Xzy
@CNRSchimie @umontpellier @enscmchimiemtp @ChemBioNAC @IBMM_Balard
pubs.rsc.org
We report the synthesis of (2-(N-acetylsulfamoyl)ethyl)carbamate (NAC)-linked thymidine dinucleotide via the sulfo-click reaction and its incorporation into DNA oligonucleotides. Thermal denaturation...
0
4
18
Visible-light-driven borylation and phosphorylation of aryl halides by phosphonium ylide organophotoredox catalysis (@ChemCommun): https://t.co/HFEPDzWmNA.
0
7
69
Well-Defined Rhodium and Iridium Complexes Containing Chiral Cyclic(Alkyl)(Amino)Carbenes for Asymmetric Hydrogenation by Rodolphe Jazzar, Joanna Wencel-Delord, Marc Mauduit, and co-workers (@Rodolphe_Jazzar, @DelordWencel) 🔓
chemistry-europe.onlinelibrary.wiley.com
A set of fourteen well-defined rhodium and iridium complexes containing various enantiopure cyclic(alkyl)(amino)carbene (ChiCAAC) ligands (>96% ee to > 99.5% ee) has been synthesized and fully...
0
9
44
Peer reviewers whose work is cited in the studies they are refereeing are significantly more likely to recommend accepting those papers than if their work is not cited, a new study has found.
cen.acs.org
New report finds that referees are more likely to recommend studies that refer to their own work
0
3
8
Merging central and axial chirality in Cyclic(alkyl)(amino)carbenes: The keystone for high enantioselectivities in Ru-catalyzed asymmetric olefin metathesis (@Dylan_Bouetard)(@Rodolphe_Jazzar)(@ChemRxiv) https://t.co/bnvEnAaE5Q
0
11
59
Probing the influence of ion-pairing on ligand-field excited-state dynamics
pubs.rsc.org
Exploration of the photophysical and photochemical properties of transition metal complexes has driven ground-breaking advancements in solar energy conversion technologies, including photoredox...
0
7
36
Recent advances in Chan-Evans-Lam-type synthesis of ionic compounds (@ChemRxiv): https://t.co/WMFq4Hu8UH.
0
9
75
Check out our new publication in @angew_chem. A new class of ylides! https://t.co/bq1DZzijs1
@m_yasirmehboob
@AilarBadri @IOC_PAS
onlinelibrary.wiley.com
The norbornane-2,6-dione framework can accommodate a carbon-carbon ylide with a zwitterionic σ-bond.
6
21
134
Spin-Orbit Effects in a Thallium Borohydride Stabilized by Coordination to Bis(diisopropylamino) Cyclopropenylidene (BAC) (@angew_chem): https://t.co/nOhGfAc9B8.
0
9
60
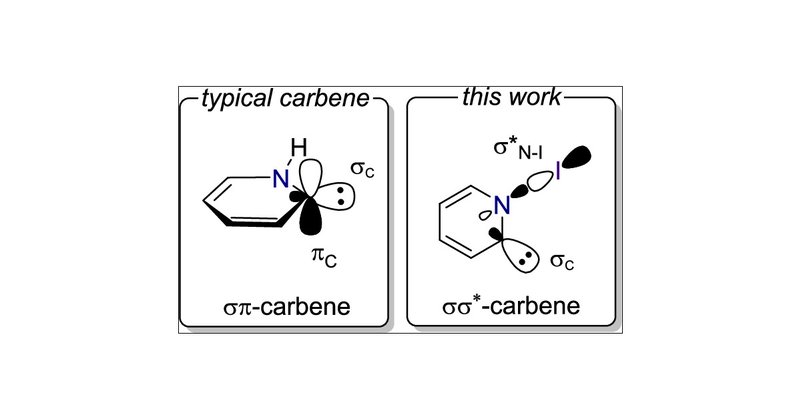
Hydrogen Activation by a σσ*-Carbene Through Quantum Tunneling | Journal of the American Chemical Society @HHU_de @uni_tue
pubs.acs.org
The electronic structure of carbenes arises from the occupation of a σ and a π frontier orbital. While parent methylene possesses a triplet ground state (σ1π1), substituents are capable of stabiliz...
0
11
76
Synthesis and Properties of Poly(vinyl chloride)-b-polyethylene Multiblock Copolymers through the Hydrodechlorination of PVC with Silylium Ions | Journal of the American Chemical Society @argonne @USC @UTKnoxville
pubs.acs.org
Block copolymers of polyethylene (PE) and polyvinyl chloride (PVC) have remained elusive materials despite PE and PVC being the first and third most produced polymers globally, respectfully. The...
0
7
34
🎇Please meet our next 10th Euroboron invited speaker - Michael Smietana🎇 Michael is Professor at the University of Montpellier, France. His current research focuses on molecular recognition and nucleic acid bioconjugates, in particular boron-modified nucleic acids.
0
6
18
The Genuine Carbene Conundrum (Jesse. L. Peltier, Rodolphe Jazzar and co-workers) #ChemEurJReviews #openaccess 🔓
chemistry-europe.onlinelibrary.wiley.com
From fleeting intermediates to well-defined molecular structures, carbenes (neutral divalent carbon) remind us that the most reactive ideas often make a large impact. Despite their wide-ranging...
0
7
54
Very pleased to share our latest publication on the Synthesis and Base-Pairing Properties of Oligonucleotides Containing 5′-(R)- and 5′-(S)-C-Aryl-thymidine now in JOC https://t.co/cS0sBPbXA3
@DebartFrancoise @VasseurJeanJacq @HausdorffMarcel @CNRSchimie @umontpellier @ChemBioNAC
1
11
31
Carbanionic reagents are not just strong bases and versatile alkylation agents—they offer much more! 🚀 Curious about the many facets of carbanion chemistry? Check out our latest review article in @NatRevChem for a deep dive into their reactivity and applications👇 @ERC_Research
Rethinking carbanion chemistry from donor substituents to weakly coordinating carbanions https://t.co/5JhlakXbuj 🧪
3
15
117
The result of a fantastic collaboration, which started a few years back with Olivier (@OBasle), Michael (@Smietana_M), Laurent (@LaurentEvanno), Yves Canac, Erwan (@erwan_poupon), and Steve (@hiltonlab) under the auspices of the ANR (@AgenceRecherche). 2/3
1
1
9
Hey peeps, Our work on the synthesis of sceptrin, ageliferin, nigramide R, chabamide and others by Photo(Flow) catalysis ( https://t.co/TVT9JqljAy) and our artificial PhotoDNAzyme ( https://t.co/DcpjVcdyje) are now on @ChemRxiv. Check them out. 1/3
1
6
34
Further exploration of the beautiful bis(borane) made in the @wpiers1 lab for small molecule activation. @LCC_CNRS
New paper out! Check out Leon's article in collaboration with DCM Grenoble published in JACS Au. The use of a bis(borane) for oxygen activation promoted by group 13 Lewis acids induces a 4-electron O2 reductive cleavage, thanks to chelation.
1
4
15
Our last contribution about photoluminescent P-ylides Ir(III) complexes in Chem. Commun., 2025, Advance Article https://t.co/9CYg6tC2g3
@OBasle@CanacYves@LCC_CNRS
pubs.rsc.org
A novel class of cyclometallated photoactive iridium(iii) complexes containing phosphonium ylide ligands has been synthesized and thoroughly characterized. Their photophysical and redox properties...
1
5
45