Steven Tong
@syctong
Followers
4K
Following
5K
Media
155
Statuses
3K
Infectious diseases physician, clinical researcher with interests in genomics and Indigenous health, follower of Jesus
Melbourne, Australia
Joined November 2010
🆕🔥State of art review article by 🌟 s @DrToddLee @syctong Navigating the Challenges in Staphylococcus aureus Bloodstream Infection: A Practical Guide to Management "Further subgroup analyses from SNAP will clarify whether the CzIE differentially impacts outcomes with cefazolin
3
78
177
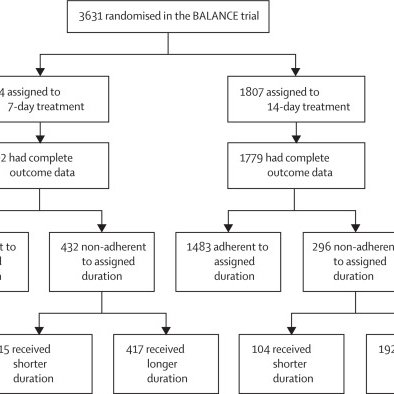
Non-adherence in BALANCE RCT to assigned duration occurred in 728 (20·3%) of 3581 patients! 🆕💫Post-hoc analysis aimed to identify factors associated with this non-adherence, and evaluate its effect on validity of trial results @BradSpellberg @DrToddLee
https://t.co/rSlAjEEcqa
thelancet.com
Non-adherence in BALANCE was significantly associated with a range of important prognostic factors, which might have introduced bias. Causal inference methods addressing this bias showed a consistent...
2
9
28
Those interested in adaptive platform trials might be interested in this recent chat @syctong and I had with @TheLancetInfDis podcast https://t.co/uXbeCH4mye
0
3
6
Giving a talk tomorrow AM about designing a trial to test antiplatelet agents for S aureus bactermia 08:00 Eastern time. @syctong
@jesusrbano
@ESCMID Register to watch https://t.co/f2z4ExYHYl
us06web.zoom.us
Zoom is the leader in modern enterprise video communications, with an easy, reliable cloud platform for video and audio conferencing, chat, and webinars across mobile, desktop, and room systems. Zoom...
2
4
14
Heard of SDSE? This under-recognised strep bacterium is causing a growing number of serious infections in Australia, with First Nations Australians disproportionately affected. Learn more 🔗 https://t.co/sQScJXe7W7
@ouli_xie @syctong @MDaviesLab
0
6
4
Another great paper from @ouli_xie. This on invasive Streptococcus dysgalactiae subsp. equisimilis (iSDSE). Many many pearls in the paper, highlighted in the thread below.
Excited to share our latest paper on invasive Streptococcus dysgalactiae subsp. equisimilis (iSDSE) disease in Australia and in a global context. @syctong @MDaviesLab
0
0
6
🔥🔥Just published in #CID world-first clinical trial of IFN-a nasal spray vs placebo reduced #COVID 🦠 incidence 📉 by 40% in cancer pts. Well tolerated, safe, effective… Full link here: https://t.co/Lds6PPpAya
1
7
18
🆕💫Adherence to Quality-of-Care Indicators and Mortality Outcomes in Patients With MRSA Bacteremia A Post Hoc Analysis of the CAMERA2 RCT #IDXposts
https://t.co/Wf2e7Rs4Bs
jamanetwork.com
This post hoc analysis of a randomized clinical trial investigates whether patient participation in clinical trials is associated with practitioner adherence to methicillin-resistant Staphylococcus...
0
9
31
We’re proud to share that the SNAP Trial Consumer Reference Group is a finalist in the 2025 #CPIRawards! Thank you to our Consumer Reference Group Members for driving meaningful change in health research. #SNAPTrial #ConsumerVoices #CoDesign
@syctong @AshaBowen @Josh_S_Davis
0
1
2
🆕💫Adjunctive Fosfomycin for the Treatment of Staphylococcus aureus Bacteremia: A Pooled Post-hoc Analysis of Individual Participant Data from Two Randomized Trials( BACSARM ( dapto) & SAFO( cloxa) @syctong
@BradSpellberg @DrToddLee #idxposts
https://t.co/YMoeHemK4c
academic.oup.com
This pooled analysis of 2 randomized controlled trials used Bayesian and frequentist approaches to evaluate adjunctive fosfomycin for Staphylococcus aureus
2
16
51
We're proud to announce the SNAP Trial has been nominated for the Consumer Partnership in Research (CPIR) Award for Outstanding Consumer Advisory Group Impact on Health and Medical Research. Vote here before 24 July > https://t.co/qgwB5bmO4J
@AshaBowen @syctong @Josh_S_Davis
0
2
13
Excellent meta-research: A trial within a large trial shows that consent form length impacts recruitment. Please consider too much text can exclude vulnerable groups / We need to rethink consent forms, too much information may harm. https://t.co/dksDmQDXl1
jamanetwork.com
In this issue of JAMA, Johansen and colleagues1 report the results of a trial that investigated the impact of digital recruitment letter formats on recruitment to a larger clinical trial, the...
1
10
26
Practicing With Intent: How to Teach an Old Dogma New Tricks It was an honor to stand beside this team of Martians and Venusians @MCPhillipsMD
@BradSpellberg
@DrEmilyMcD
@ABsteward
@DrToddLee
@syctong & many others https://t.co/V8mPuBbL5g
academic.oup.com
Abstract. Clinicians are constantly bombarded with an onslaught of newly published data, yet they must make clinical decisions despite a dearth of clinical
1
8
26
Making sense of hierarchical composite endpoints in randomized clinical trials ? a primer for infectious disease clinicians and researchers ✅ Just Accepted ✍️ @seanongwx @syctong 🔗 https://t.co/gC9X4xRfP8
0
6
8
@snap_trial is the little engine that could! Congratulations on passing 4500 participants @syctong @Josh_S_Davis
4
4
33
Amazing contribution Owen to the @snap_trial community! The whole study only works because of your kind of engagement.
Yesterday my team and I enrolled the 500th patient in SNAP trial between RPH and FSH (50/50 platform/registry). So proud to be part of such an amazing project which will change the way we treat S aureus bacteremia @SNAPtrial @Josh_S_Davis @syctong
0
1
17
🆕💫 Systematic Review and Meta-Analysis @CMIJournal @ConnorProsty @DrToddLee @DrEmilyMcD @syctong 30 observational studies( SNAP & CloCeBa not included) N: 3869 Cefazolin versus Antistaphylococcal Penicillins for the Treatment of MSSA Bacteremia #idxposts
https://t.co/R01Dm8E2op
clinicalmicrobiologyandinfection.org
There is debate on whether cefazolin or antistaphylococcal penicillins should be the first-line treatment for methicillin-susceptible Staphylococcus aureus (MSSA) bacteraemia. Ongoing trials are...
7
22
54
Annual N95 fit-testing is still required based on 1 small study of 229 participants. In a study of 12,065 HCWs followed over 3 yrs, fit‑test failure was <0.5% (Martin et al). Annual fit‑testing still costs US healthcare ~$200–400 M/yr. Time to switch to every 3 years.
4
7
37
Thanks @PaulSaxMD for highlighting @snap_trial ! @TheDohertyInst @ESCMID @UniMelbMDHS #IDTwitter @Josh_S_Davis
The SNAP trial may finally resolve some of the longest-running debates in SAB management (cefazolin vs semi-synthetic PCN? Is PCN really active vs PSSA?). Congrats to @syctong @Josh_S_Davis, and the global team who made this ambitious trial happen.
0
0
20
It’s happening now! The results of the SNAP PSSA and MSSA domains Recently, the number of patients recruited in SNAP surpassed all randomized patients in staph aureus trials combined! #ESCMIDGlobal2025
@syctong @DrToddLee
2
9
70