Saad Nagi
@saadsnagi
Followers
409
Following
2K
Media
10
Statuses
670
Neuroscientist. PI @CSAN_LiU, Linköping Univ., Sweden. #Pain. #Touch. #Microneurography. Adjunct @westernsydneyu
Linköping, Sweden
Joined April 2011
1/12 Excited to share our new paper, “Leveraging Deep Single-Soma RNA Sequencing to Explore the Neural Basis of Human Somatosensation,” now out in @NatureNeuro! 🎉
nature.com
Nature Neuroscience - Dorsal root ganglia (DRGs) contain a plethora of neuron types. The authors show that the existence of human-specific DRG neuronal types and microneurography recordings reveal...
5
21
65
9/9 This work was supported by @nhmrc, @arc_gov_au, @Vetenskapsradet, and @sls_sv, and institutions @UNSW, @neuraustralia, @westernsydneyu, and @liu_universitet @CSAN_LiU.
0
0
0
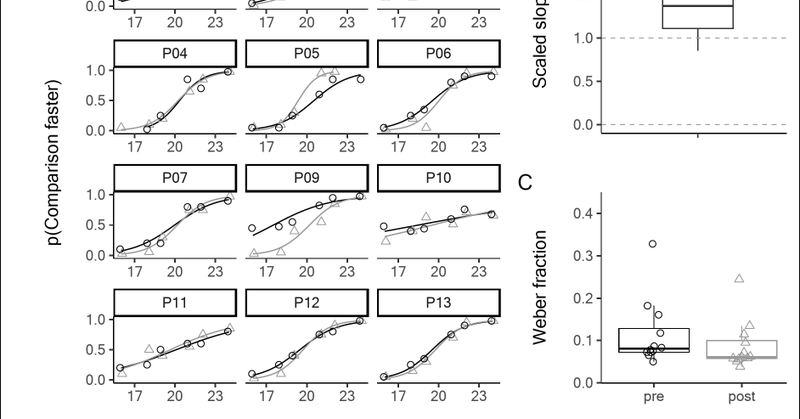
8/9 Our findings challenge the channel theory by showing that receptors specialized for low-frequency signaling are not essential for flutter-range perception. This suggests how frequency constancy can be achieved across skin regions, regardless of the afferent type activated.
1
0
0
7/9 Despite reliance on vibration transmission to activate remote PCs, we found that flutter-range frequency discrimination was unimpeded across both skin types. Comparisons with stimuli applied to the contralateral side also indicated that perceived frequency was unaffected.
1
0
0
6/9 Exp. 1 tested freq. discrimination on the ulnar styloid (hairy skin) before/after blocking local FA1 inputs with anesthesia. Exp. 2 compared freq. perception on anesthetized vs. non-anesthetized hands. Exp. 3 used A-fiber compression block affecting digital glabrous skin.
1
0
0
5/9 We predicted that when local touch sensation is abolished by either anesthetic injection or compression block, vibration discrimination performance would persist due to vibration transmission to FA2 afferents outside the affected area.
1
0
0
4/9 The FA2-PC complex is also uniquely sensitive to distant sources of vibration. This led us to explore how remote skin sites contribute to vibrotactile perception. In particular, we focused on flutter-range vibrations propagated to skin sites remote from the point of contact.
1
0
0
3/9 Our earlier work showed that low-amplitude pulsatile stimuli in the flutter range (20–40 Hz) can recruit FA2 afferents, producing a low-frequency percept of vibration similar to that associated with FA1 fibers ( https://t.co/TeQQhA5dzX).
elifesciences.org
Perception of vibrotactile frequency depends on the neural discharge pattern rather than the afferent type, thus requiring a reevaluation of the notion of Pacinian/non-Pacinian channels in tactile...
1
0
0
2/9 The “channel theory” of vibrotactile perception suggests different afferent classes signal specific frequency ranges: Fast-adapting type 1 (FA1) fibers signal low frequencies (<50 Hz), while FA2, associated with Pacinian corpuscles (PCs), signal higher ones (50–300 Hz).
1
0
0
1/9 Happy to share our new paper, “Contribution of remote Pacinian corpuscles to flutter-range frequency discrimination in humans,” now out in @SciReports! A collaborative effort with @somacrat, Kevin Ng, David Mahns, Ingvars Birznieks, & Richard Vickery.
nature.com
Scientific Reports - Contribution of remote Pacinian corpuscles to flutter-range frequency discrimination in humans
1
0
3
12/12 This work was supported by funders @NIH HEAL U19, @Vetenskapsradet, @KAWstiftelsen, @ERC_Research, and @regionost, and institutions @Penn, @karolinskainst, and @CSAN_LiU @liu_universitet.
0
0
2
11/12 In summary, we generated a comprehensive hDRG neuron atlas using LCM-based single-soma deep RNA sequencing and spatial transcriptomics, providing unique predictions into human somatosensory physiology, confirmed by microneurography.
1
0
2
10/12 In C-fiber recordings: As predicted by molecular profiling, we found C-LTMRs that responded to heating and capsaicin but not to menthol. All C-LTMRs responded to cooling, indicating a non-TRPM8 cooling mechanism.
1
0
1
9/12 In A-fiber recordings: As predicted by molecular profiling, A-LTMRs (Field type) did not respond to heating or cooling. Further, we found two A-HTMR types: one responsive to cooling, one not; neither responded to heating.
1
0
1
8/12 Based on gene profiles, we predicted temperature sensitivity in A- and C-fiber low-threshold and high-threshold mechanoreceptors (LTMR and HTMRs), testing these predictions in awake healthy participants using single-unit microneurography recordings.
1
0
1
7/12 Spatial transcriptomics also revealed previously unidentified spatial clustering patterns: different hDRG neuron types were intermingled in a DRG section without obvious spatial segregation; however, neurons within each type exhibited spatial clustering.
1
0
1
6/12 10x Xenium spatial transcriptomics confirmed the expression of top marker genes and identified 16 hDRG neuron populations, matching those identified by our deep single-soma RNA sequencing.
1
0
2
5/12 Gene expression patterns in DRG neurons were generally more similar between human and macaque than between human and mouse, with several genes uniquely expressed in hDRG neurons.
1
0
1
4/12 Cross-species cell-type analysis comparing human, mouse, and macaque DRG neurons revealed divergence in potential pain-sensing neurons, suggesting the possible existence of neuron types unique to humans.
1
0
2
3/12 We developed a laser capture microdissection (LCM)-based approach for transcriptomic profiling of human dorsal root ganglia (hDRG) neurons, identifying 16 molecularly distinct neuron types.
1
0
1
2/12 Spearheaded by @WenqinLuo with lead postdoc @HuashengY, this work was a collaborative effort with @PatrikErnfors, @OlaussonHakan, and my team.
1
1
3