Ritter Lab
@ritter_lab
Followers
11K
Following
2K
Media
38
Statuses
376
Research in the Ritter group focuses on the development of novel reaction chemistry.
MPI KoFo, Mülheim an der Ruhr
Joined August 2017
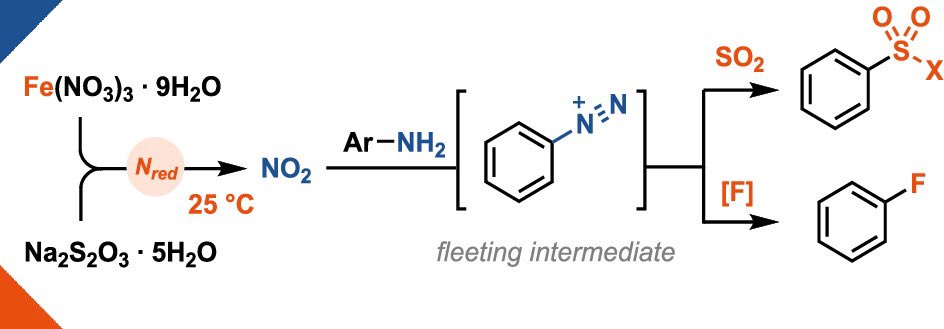
We are happy to present our newest publication in @ScienceMagazine about safer aryldiazonium chemistry!. Nitrate is reduced to generate aryldiazoniums as fleeting intermediates for the direct conversion of anilines and aminoheterocycles to arylhalides.
science.org
Aryldiazonium salts remain a staple in organic synthesis and are still prepared largely in accord with the protocol developed in the 19th century. Because of the favorable reactivity that often...
21
62
368
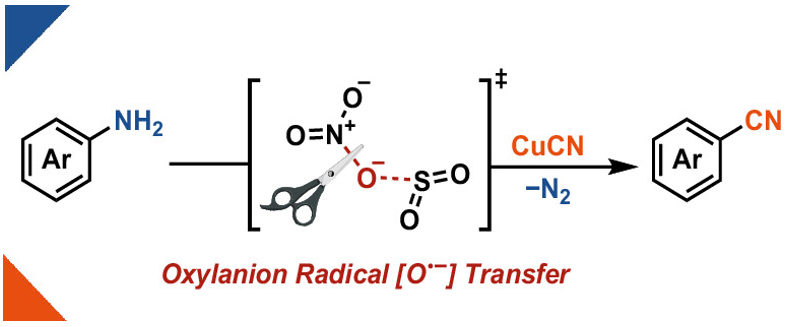
In our new @JOC_OL paper, we show the deaminative cyanation of anilines using our nitrate reduction approach along with a theoretical study that points towards an oxylanion radical transfer mechanism that generates NO2 from nitrate!. Open access! 🔓.
3
23
262
Selenoxide 🤝 DNA-encoded libraries! 🧬 Out now in @NatureChemistry, we present a selenium linchpin that can be introduced site-selectively into DNA conjugates and subsequently functionalized!. Open access! 🔓.
3
17
194
Nucleophilic substitution at a neopentyl-like electrophile ⁉️ Our new, bench-stable, bifunctional Iodo-BCP-TT reagent makes it possible! Our new, highly modular platform to access a broad range of BCP isosteres is now out in @NatureSynthesis!. ⬇️⬇️⬇️.
3
18
180
RT @NatureSynthesis: Now online & open access:. Article by Zibo Bai, Zikuan Wang, Thomas Hin-Fung Wong & Tobias Ritter @ritter_lab . Thiant….
nature.com
Nature Synthesis - The lack of stable and versatile bicyclo[1.1.1]pentyl reagents hinders their broader adoption as aryl bioisosteres in drug discovery. Now, a stable, bifunctional...
0
10
0
After a multi-year journey to bring thianthrenation-like chemistry to water, we now present a selenoxide reagent that can be used to perform single atom modifications selectively on tyrosine residues in proteins or peptides! . Out now in @NatureChemistry!.
6
37
332
Kharasch addition but with a little twist 🪄 In our new @J_A_C_S paper, the use of a phosphine-ligated Cu-complex enables the use of unstabilized, nucleophilic alkyl radicals and electron-deficient alkenes for a Kharasch-type haloalkylation reaction!. 🔓.
3
22
175
New @angew_chem article alert! 👀 We expand the chemical space accessible from BCP-TT reagents via a reductive cross-coupling reaction with alkyl bromides using copper/photoredox catalysis!. Open access 🔓.
5
20
211
RT @J_A_C_S: Iron-Mediated Nitrate Reduction at Ambient Temperature for Deaminative Sulfonylation and Fluorination of Anilines | Journal of….
pubs.acs.org
Preparation of arylsulfonic acids and derivatives can be achieved under mild conditions from aryldiazonium salts, although conventional methods often require isolation or accumulation of these...
0
13
0
RT @TimSchu34938298: The nitrate reduction saga continues. Very happy that the 2nd follow-up paper of our nitrate reduction strategy is….
pubs.acs.org
Preparation of arylsulfonic acids and derivatives can be achieved under mild conditions from aryldiazonium salts, although conventional methods often require isolation or accumulation of these...
0
11
0
Nitrate reduction at 25 °C? A little iron is all it takes! 🪄 The use of cheap iron(III) nitrate (30€ per kg!) enables the in-situ generation of aryldiazonium salts which can be engaged in sulfonylation & fluorination reactions!. Out now in @J_A_C_S 🔓.
3
37
231
Nitrate reduction meets palladium – in our new @angew_chem paper, we show how the generation of aryl diazonium salts as fleeting intermediates via nitrate reduction can be coupled with Pd-catalysis in a Suzuki-type cross coupling reaction!.
1
16
186
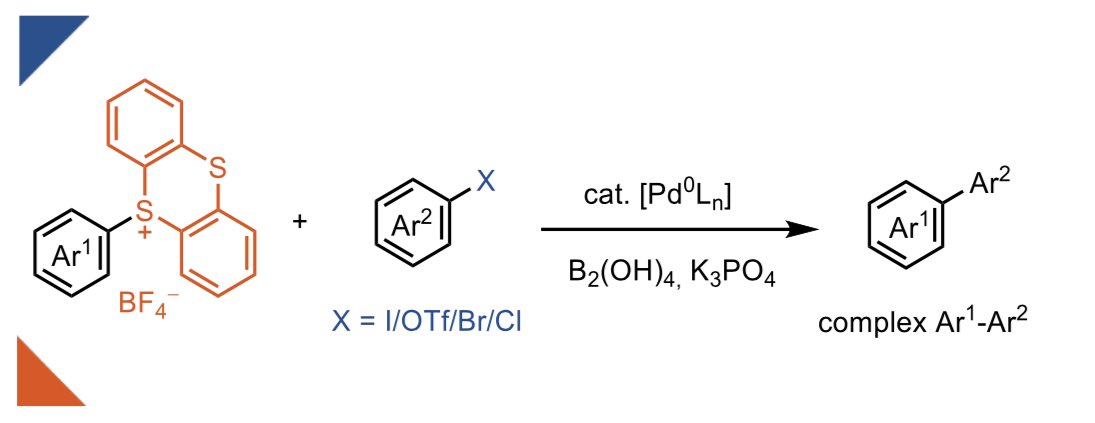
In our new publication in @angew_chem, we demonstrate how the ultra-fast rate of oxidative addition of aryl-TTs to Pd(0) can be used to achieve cross-electrophile coupling with aryl-I/Br/OTf/Cl! . Open access 🔓!.
4
16
182
RT @MateosLab: 🚨 PhD Opportunity Alert! RT appreciated!. A fully funded PhD position is available in the Mateos Group at the University of….
0
23
0
RT @CornellaLab: 🚨Calling medicinal chemists🚨: .A Suzuki het-het coupling sometimes does not require complex ligands. An air stable Ni wou….
0
26
0
RT @J_A_C_S: N-Protonated Acridinium Catalyst Enables Anti-Markovnikov Hydration of Unconjugated Tri- and Disubstituted Olefins | Journal o….
pubs.acs.org
The preparation of alcohols with anti-Markovnikov selectivity directly from olefins and water is a sought-after reaction due to its atom-economy and potential cost-effectiveness. Herein, we present...
0
11
0
Fresh off the press at @J_A_C_S ! 🚨 We present the next chapter in our development of N-protonated acridinium photocatalysts! A powerful yet stable photo-oxidant, our catalyst can reach un-conjugated olefins with oxidation potentials of up to ~2.35 V!.
6
13
246
RT @J_A_C_S: Standardized Approach for Diversification of Complex Small Molecules via Aryl Thianthrenium Salts | Journal of the American Ch….
pubs.acs.org
Thianthrenation is a useful strategy for the late-stage diversification of complex small molecules owing to the positional selectivity and the synthetic versatility of thianthrenium salts as electr...
0
10
0
'How do I thianthrenate X?' – In our new article in @J_A_C_S, @DilgamAhmadli presents simple guidelines for selecting reaction conditions and with our collaborators from @syngenta developed a robust diversification platform for further functionalizations!.
6
21
218