Qi Chen
@qichen_lab
Followers
2K
Following
11K
Media
81
Statuses
1K
Associate Professor @UUtah @UofUMedicine | sperm RNA-mediated epigenetic inheritance, embryo development, small RNAs, RNA modifications & diseases
Salt Lake City, UT
Joined November 2015
Great to see our PANDORA-seq https://t.co/lVPMynvWBi & Perspective on the exploration of small RNA universe https://t.co/T9XHRqjIqR both highlighted @NaturePortfolio among the collection of key methods & resources of the noncoding RNA field👇 https://t.co/Z4VmbVTlMU
nature.com
Research interest is growing in profiling noncoding RNAs and understanding their biological functions in health and disease contexts.
4
16
99
Perhaps our boldest hypothesis… we propose a model for RNA Structural Memory propagation @NatureCellBio, where RNA conformation is reshaped by stress, locked by RBPs, copied prion-like in condensates & passed across generations—all without altering DNA.🧵 https://t.co/Gp74O8vLVJ
2
19
73
The model starts with RNA folding energy & a multistable landscape. Under stress, RNA can adopt a new shape—and if RNA-binding proteins “lock” it in, that shape becomes a molecular memory of the stress. Think Waddington’s epigenetic landscape—but for RNA folding energy valleys.
2
3
13
The heart of the hypothesis: copying RNA shape. Some RNAs can act as both template & catalyst, guiding new RNAs with the same sequence to fold the same way. RNA alone is unstable, but RBPs stabilize the template, & condensates boost RNA–RNA interactions—as a microreaction chamber
1
2
14
Memory propagation in cells — and across generations: Newly folded RNAs bind stress-linked RBPs to assemble new condensates, passed on during cell division. Even daughter cells never facing the original stress can inherit the RNA structural memory – and its stress-adapted traits.
1
2
5
Why the memory fades eventually - as often seen in epigenetic inheritance: Two potential exits: Active reset:when conditions normalize, condensates dissolve, RBPs shift, RNA refolds. Passive fade:cells with stress-memory may grow slower, and are outcompeted over generations.
1
2
14
This animated version looks nice 😀 https://t.co/Gp74O8vLVJ
Perhaps our boldest hypothesis… we propose a model for RNA Structural Memory propagation @NatureCellBio, where RNA conformation is reshaped by stress, locked by RBPs, copied prion-like in condensates & passed across generations—all without altering DNA.🧵 https://t.co/Gp74O8vLVJ
0
2
9
Proud to work with a brave team @cai000chen @happy_jjcc @XudongZhang_RNA @tongzhoulab—thinking fearlessly to bring this piece together. In a time of uncertainty, may this idea be a seed for spring… We’ll see what evidence we and the community can bring, to confirm or refute…
0
1
2
Beyond inheritance, the model may also apply to cancer & neurodegeneration, where abnormal RNA structures could shape pathological cell states. Perhaps misfolded RNAs — not just amyloid proteins — are the central structural entity driving disease.
1
1
4
Why the memory fades eventually - as often seen in epigenetic inheritance: Two potential exits: Active reset:when conditions normalize, condensates dissolve, RBPs shift, RNA refolds. Passive fade:cells with stress-memory may grow slower, and are outcompeted over generations.
1
2
14
Memory propagation in cells — and across generations: Newly folded RNAs bind stress-linked RBPs to assemble new condensates, passed on during cell division. Even daughter cells never facing the original stress can inherit the RNA structural memory – and its stress-adapted traits.
1
2
5
The heart of the hypothesis: copying RNA shape. Some RNAs can act as both template & catalyst, guiding new RNAs with the same sequence to fold the same way. RNA alone is unstable, but RBPs stabilize the template, & condensates boost RNA–RNA interactions—as a microreaction chamber
1
2
14
The model starts with RNA folding energy & a multistable landscape. Under stress, RNA can adopt a new shape—and if RNA-binding proteins “lock” it in, that shape becomes a molecular memory of the stress. Think Waddington’s epigenetic landscape—but for RNA folding energy valleys.
2
3
13
Perhaps our boldest hypothesis… we propose a model for RNA Structural Memory propagation @NatureCellBio, where RNA conformation is reshaped by stress, locked by RBPs, copied prion-like in condensates & passed across generations—all without altering DNA.🧵 https://t.co/Gp74O8vLVJ
2
19
73
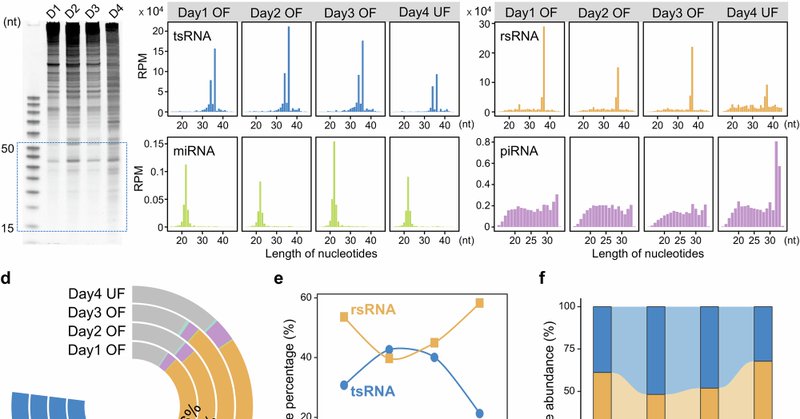
We identified tsRNAs and rsRNAs in uterine fluid, which might transmit high-fat dietary information to early embryo, influencing pregnancy outcomes and offspring health.
nature.com
Nature Communications - Uterine fluid supports early embryo development. This study reveals maternal high-fat diet alters tsRNA/rsRNA profiles in uterine fluid, leading to abnormal embryonic...
0
5
27
We’re excited to share our latest manuscript exploring a fascinating aspect of tRNA biology—how RNA processing interfaces with nucleoside modifications. Just out on @NatureComms 🔬 A wonderful collaboration with @GlattSebastian
https://t.co/sJLbANbDqh
nature.com
Nature Communications - tRNA modifications are essential for function, yet their timing relative to processing is unclear. Here, the authors show that queuosine and derivative modifications occur...
2
9
30
New preprint from the lab! With @Brumbaugh_JB, we show that P-bodies sequester developmentally relevant RNAs to influence cell fate. miRNAs direct this conserved regulatory process, and manipulating it enhances totipotency and germ cell programming.
biorxiv.org
Recent studies have emphasized the significance of biomolecular condensates in modulating gene expression through RNA processing and translational control. However, the functional roles of RNA...
0
6
24
Very glad to see our Sequential Activation Hypothesis highlighted on the cover @TrendsBiochem, linking small RNA-TLR interactions to autoimmune disease mechanisms.
How can abnormal small RNA biogenesis spark autoimmune disease? Our opinion @TrendsBiochem discuss a novel two-stage activation hypothesis that may explain why autoimmunity hits women harder, via small RNA-TLR7/8 interactions & augmented by autoantibodies. https://t.co/DXr8PmPhSB
2
6
35
We put together a step-by-step protocols for PANDORA-seq, now available in @NatureProtocols, including the sample preparation for sperm, sperm heads; and the analyzing software SPORTS optimized for various types of small RNAs including tsRNAs, rsRNAs, and miRNAs.
1
6
41
Proud of the team @happy_jjcc @XudongZhang90 @cai000chen @tongzhoulab for thinking fearlessly to put the ideas together!
0
1
5
This idea is driven by advanced sncRNA sequencing (e.g. PANDORA-seq), uncovering an expanding universe of tsRNA/rsRNA/ysRNA & more https://t.co/T9XHRq27zj These sncRNAs go beyond RNAi, adopting aptamer-like roles by binding TLR7/8 to shape immune responses https://t.co/ZZPNBfbNol
1
0
3