Mateo Aboy
@mateo_aboy
Followers
250
Following
457
Media
11
Statuses
733
Director of Research @Cambridge_Uni. #Data, #IP, #Patents, #Privacy, #GDPR, #biotech. Tweets are my own.
Cambridge, England
Joined October 2017
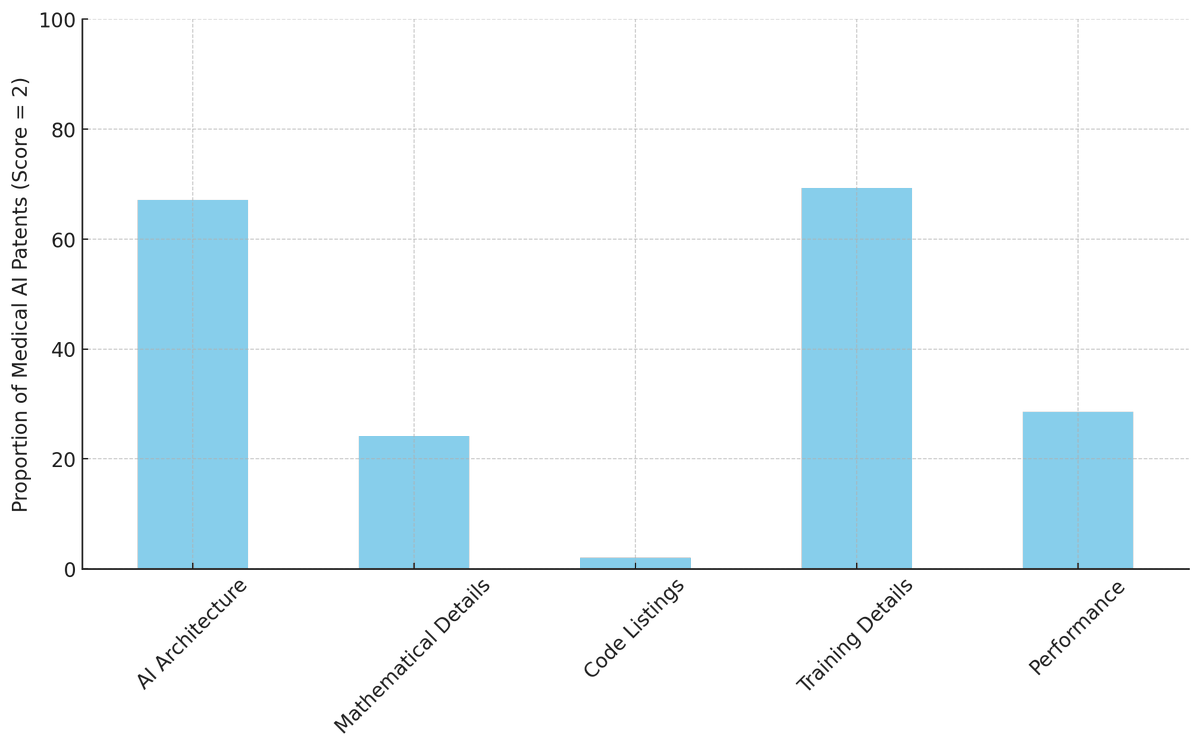
Hot off the press in @NatureBiotech "The Sufficiency of Disclosure of Medical AI Patents with @WNicholsonPrice @CeBIL_Center @cambridgelaw. Full text at: or (1) To what extent are medical AI patents disclosed? How does this level
2
8
9
RT @TiMinCeBIL: So excited about our papers published this week:. #1 in @NatureMedicine: #Data Sharing in #Precision #Medicine: https://t.c….
0
1
0
RT @PeterSinger: Like some ethical guidelines for using LLMs like ChatGPT? My former student @JulianSavulescu and team provide a practical ….
nature.com
Nature Machine Intelligence - In this Comment, we propose a cumulative set of three essential criteria for the ethical use of LLMs in academic writing, and present a statement that researchers can...
0
4
0
RT @juliansavulescu: My team and I provide clear guidelines in NatureMachIntel to using LLMs ethically in research. A practical framework f….
nature.com
Nature Machine Intelligence - In this Comment, we propose a cumulative set of three essential criteria for the ethical use of LLMs in academic writing, and present a statement that researchers can...
0
13
0
Check out our just-published Nature Machine Intelligence @NatMachIntell "Guidelines for ethical use and acknowledgement of large language models in academic writing"
1
5
7
RT @TiMinCeBIL: Happy to see our 2 papers in new @JIPLP:. 1. The sufficiency of disclosure of #AI invention, with @mateo_aboy , @AparajitaL….
academic.oup.com
Abstract. The complex and data-driven nature of artificial intelligence (AI) raises questions for the sufficient disclosure of patent applications in this
0
4
0
RT @AparajitaLath: Excited to share my article in JIPLP with @mateo_aboy, @TiMinCeBIL and K. Liddell on a hot-topic - Sufficiency of Disclo….
academic.oup.com
Abstract. The complex and data-driven nature of artificial intelligence (AI) raises questions for the sufficient disclosure of patent applications in this
0
4
0
RT @EthicsPolicyLab: What is the impact of the EU's AI Act on AI- and ML-enabled medical devices? New paper out in @npjDigitalMed! 📣. @Effy….
nature.com
npj Digital Medicine - The newly adopted EU AI Act represents a pivotal milestone that heralds a new era of AI regulation across industries. With its broad territorial scope and applicability, this...
0
1
0
RT @npjDigitalMed: The new EU AI Act introduces strict regulations for AI systems, notably impacting the #digitalhealth sector. This pers….
0
1
0
RT @TiMinCeBIL: Delighted to see our new paper on „Navigating the #EU #AI #Act: implications for regulated #digital #medical #products“ wit….
nature.com
npj Digital Medicine - The newly adopted EU AI Act represents a pivotal milestone that heralds a new era of AI regulation across industries. With its broad territorial scope and applicability, this...
0
8
0
RT @TiMinCeBIL: 📣 Our new paper in @ScienceMagazine #Translational #Medicine with @johnliddicoat, A. Hamidzadeh, K. Liddell, M. Schito, @da….
science.org
New government drug repurposing programs in the United States and Europe present new opportunities for stakeholders.
0
2
0
RT @classicaproject: 📣 Publication news! How can we balance privacy & accessibility in health data transfers post-Schrems II?. 📄 "Supplemen….
0
5
0