Ingmar Schäfer
@ingmarschafer
Followers
147
Following
2K
Media
0
Statuses
106
structural biology @ZML_Dresden @PLID_info the other places: @[email protected]; @ingmarschafer.bsky.social
Joined June 2017
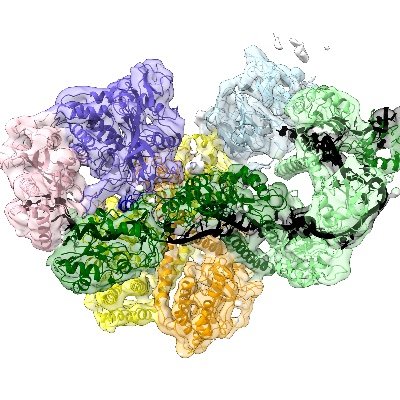
Excited to share our latest research on co-translational mRNA decay by the exosome in human cells! We uncover how the exosome-SKI complex is recruited to ribosomes and propose a novel role for SKI in recognizing collided disomes. @MPI_Biochem 👇👇👇
4
52
221
The paper was published in @Nature and can be downloaded here:
nature.com
Nature - The structure of a human exosome–ribosome supercomplex reveals the mechanisms behind the formation of active cytoplasmic exosomes and their role in co-translational RNA decay.
3
19
70
Heartfelt congratulations to @ingmarschafer on his promotion to Professor! Your presence will be greatly missed, but we are excited for the impact you will continue to make in your new position. Wishing you nothing but the best on your journey ahead! 🎉🥳🚀 #PLID #ZML #Dresden
BIG NEWS No 2 🥳🥳🥳 ! Together with @PLID_Info @DiabResearch @HelmholtzMunich @tudresden_de and @Medizin_TUD As of April 1st @ingmarschafer of @LabConti and @MPI_Biochem is appointed as Professor and Chair of Structural Membrane Biology at PLID and ZML.
3
4
25
Have a look at our new manuscript! Multiple conformational states of the Ski complex reveal a direct interaction of Ski2 with the exosome during RNA handover. Congratulations all! 👇👇👇
Happy to share our latest work on cytoplasmic mRNA degradation by the Ski-exosome assembly. We reveal via SPA cryoEM how different conformational states of the Ski complex are connected to exosome recruitment and RNA handover. Thanks to everybody involved @LabConti @MPI_Biochem
0
10
88
Happy to share our latest work on cytoplasmic mRNA degradation by the Ski-exosome assembly. We reveal via SPA cryoEM how different conformational states of the Ski complex are connected to exosome recruitment and RNA handover. Thanks to everybody involved @LabConti @MPI_Biochem
4
22
107
Our new study exploring the molecular principles governing packaging of eukaryotic mRNPs has just come out in @GenesDev . We find evidence for RNA packaging and compaction relying on positively charged IDRs of potentially many RNA binding proteins. Interested? Check it out!
5
42
220
Excited to share new work on the human CCR4-NOT complex: our analysis pinpoints the N-terminal CNOT1-CNOT10-CNOT11 module as a conserved interaction platform. We identified GGNBP2 - a tumor suppressor and spermatogenic factor - as a conserved binding partner. @MPI_Biochem
2
11
60
Very happy that this story (a team effort by @Grant49715112, @a_machadoamorim, @VinneMacc and others) is out! What was initially one strange observation is now a report of how a helicase dissociates from its RNA substrate upon activation. Warning: no mechanism proposed!
The NMD factor UPF2 is an activator of the RNA helicase UPF1. However, binding to UPF2 releases UPF1 from RNA. Lack of a stable UPF1-UPF2-RNA complex highlights the transient nature of this UPF1-mRNP https://t.co/ol5rZTkFkv
4
3
39
We have a PhD position! This is a very cool interdisciplinary project and you will get to work with great collaborators. Join us to examine the life cycle of immune signalling condensates! DM or email if interested. More info in the link below.
10 PhD positions in infection biology & immunology related disciplines International Max Planck Research School for Infectious Diseases and Immunology (@mpiib_berlin), Berlin, Germany https://t.co/DL2Vcw7cPZ
#ScienceJobs #PhDbiology #PhDBerlin
1
5
6
Happy to announce that we have open PhD and PostDoc positions at the BZH @UniHeidelberg. Interested in how #mitochondria are shaped and why this is important for their function? Wanna play with membranes and proteins to reconstitute mitochondria @SynCellEU? Send us a DM Please RT
1
26
37
Biochemically validated structural model of the 15-subunit IFT-B complex
biorxiv.org
Cilia are ubiquitous eukaryotic organelles important to cellular motility, signalling and sensory reception. Cilium formation requires intraflagellar transport for trafficking of structural and...
5
10
57
In this manuscript we used @alphafold to predict the structure of the 15-subunit IFT-B complex. The structural model was verified using a bunch of biochemical and biophysical techniques.
1
2
15
What do the visual arts, such as sculpture, have in common with structural biology? Structural biologist and Cytiva customer, Sebastian Falk explains. https://t.co/NYgBkoFp8f
#DiscoveryMakers
#WeAreCytiva
0
1
8
Happy to share our work on the human SKI complex which adopts distinct conformational states to recognize and extract ribosome-bound RNA. These functional states regulate access of the RNA 3' end to the cytoplasmic exosome for co-translational degradation. Congratulations to all!
7
39
250
Because of the most recent data from our collaboration partner @DannyHChou and the independent data of @PoulNissen we decided to also upload tentative!! model of the human insulin receptor ectodomain bound by three insulin
rcsb.org
tentative model of the human insulin receptor ectodomain bound by three insulin
4
4
9
Welcome Kirby Swatek, whose lab at MRC PPU is now open! For further information on Kirby's research and available positions see here https://t.co/OMuNzUNiJI
#ubiquitylation #ubiquitin
0
5
50
Sheepishly presenting the very first preprint from our lab @FUBiochemistry @fubcp Intrinsically disordered regions of Tristetraprolin and DCP2 directly interact to mediate decay of ARE-mRNA
biorxiv.org
The RNA binding protein Tristetraprolin (TTP) is a potent activator of mRNA decay, specifically for transcripts bearing AU-rich elements (AREs) in their 3′-untranslated regions. TTP functions as a...
6
8
31