Kerstin Noëlle Vokinger
@KNVokinger
Followers
461
Following
612
Media
9
Statuses
124
Professor of Law and Medicine at University of Zurich and ETH Zurich Academic Chair for Regulation in Law, Medicine, and Technology
Joined April 2019
In a new Perspective, Kerstin N. Vokinger, MD, JD, PhD, Guanqiao Li, MD, and Olivier J. Wouters, PhD (@ojwouters), write that China has recently emerged as an important player in new drug development — a field traditionally dominated by the United States and Europe. This shift
0
9
30
Viewpoint from @james_hillis, Edward Scheffer Cliff, and @KNVokinger: The FDA's recent authorization of an AI-powered medical device for dementia prognosis marks a step in the regulation and clinical implementation of #AI in healthcare. https://t.co/YIcO4GHiKH
0
5
13
New study @TheLancet WHO shapes priorities for the world’s most influential medicines list? We analyzed applicants for WHO essential medicines list and discuss solutions to improve access to essential medicines https://t.co/g540PARiu8 w/ @kjmeetswrld @CamilleGla52655
0
4
12
Was sie mit den steigenden Krankenkassenprämien zu tun hat: Die Angstgegnerin – eine Professorin legt sich mit der Pharmabranche an
nzz.ch
Der Anstieg der Krankenkassenprämien hat einiges mit neuen teuren Medikamenten zu tun. Kerstin Noëlle Vokinger, Professorin an der Universität Zürich, sagt allerdings: «Es gibt keinen messbaren...
0
2
6
🗞️Kerstin Noëlle Vokinger: ‘New cancer therapies are being approved for patient groups not included in clinical trials’ Read more 👇
english.elpais.com
Researchers from the universities of Zurich, Yale and Harvard reveal the differences between the characteristics of the patients who receive the drug during pharmaceutical testing and those who end...
0
1
8
Kerstin Noëlle Vokinger: “Las nuevas terapias contra el cáncer son aprobadas para perfiles de pacientes no incluidos en los ensayos” Investigadores de Zurich, Yale y Harvard revelan diferencias que pueden reducir la seguridad y eficacia https://t.co/88VBS17AwT vía @el_pais
elpais.com
Investigadores de las universidades de Zúrich, Yale y Harvard revelan diferencias entre las características de los enfermos en los que los fármacos son probados y aquellos que los acaban recibiendo,...
0
4
8
New study @AnnalsofIM We assessed time from approval until reimbursement of new drugs in US, Germany, England, France, and Switzerland (2011-2022) @CamilleGla52655, @miquelsb2, @DusetzinaS With coverage @NZZ
https://t.co/BZ03wZvk5C
nzz.ch
Laut der Pharmaindustrie hemmen tiefe Preise und lange Preisverhandlungen den Zugang für Schweizer Patienten zu Medikamenten. Politik und Behörden drängen dagegen auf weitere Preissenkungen.
A new report finds that the U.S. lags behind European countries in time from drug approval to reimbursement. This is important because while approval enables market entry of new drugs, insurance reimbursement is what determines patient access: https://t.co/k0btjuWrsI
0
4
13
Drug prices declined substantially after patent expiration across 8 high-income countries, from 30% to 80% during the 8 years after patent expiration. https://t.co/Fd2xD0bYsT
@KNVokinger
0
11
8
Viewpoint compares use and costs of GLP-1 receptor agonists for weight loss between the US and 3 other peer countries. https://t.co/HO4SVel4ls
0
6
10
Happy to share our new perspective article in @TheLancetOncol highlighting shortages of #essential (and #curative) #cancer medicines https://t.co/XGNvXp0ksq Kudos to amazing co-authors @kjmeetswrld @KNVokinger
@ImperialSandC @UZH_en @LSEHealthPolicy @imperialcollege
0
7
20
Neujahr ist die Zeit für neue Regeln – jedes Jahr treten Hunderte davon in Kraft. Doch wer ist dafür verantwortlich, und wo wird am meisten vorgeschrieben? Ein Zürcher Forschungsteam hat dies untersucht. Von @DanielGerny
https://t.co/8yp0nwrmZg
0
1
5
Global competition between countries on the successful development of AI technologies has emerged over the past decade, with medicine at the forefront of interest. This study finds that China dominated in terms of absolute number of trials. Read more: https://t.co/OptrI0yk0E
1
3
20
New study geographic representation of trials for AI med devices @NEJM_AI: 97% national. Approx half in China, followed by US & Japan https://t.co/8iPIeYu11u. Funded by @snsf_ch Covered by Swiss National News @srfnews @georg_halter w/ @FrauenfelderTh
https://t.co/JyGZJxBDUc
srf.ch
Fast alle KI-Medizinprodukte sind nur in einem Land trainiert worden. Das zeigt eine neue Studie und warnt vor Risiken.
0
1
13
Thinking the future of essential medicines @WHO. Great thoughts with incredible talents and scientific superstars
2
11
47
New study @AnnalsofIM: Review duration of drugs longer&applications later submitted in Europe vs US-patients benefit if time difference in submission is further minimized @miquelsb2, @CamilleGla52655, @thomasmd, @akesselheim,@PORTAL_Research,@BWHUrology
https://t.co/Jh5KKVeSRi
acpjournals.org
The speed of drug regulatory agencies in the United States and Europe is often a source of discussion. The objective of this research was to assess regulatory review duration of first and supplemen...
1
4
13
New study @LancetDigitalH: Analysis of 510(k) predicate network of AI medical devices. Tasks of AI medical devices change over time and may raise safety issues. https://t.co/sw7odZkIZO
@Captcoma, @cxbln, @Unispital_USZ funded by @snsf_ch covered by @NZZ
Auch in Spitälern hält KI Einzug. Eine Medizinerin macht auf Risiken aufmerksam und kritisiert den Plan, amerikanische Medizinprodukte automatisch auch in der Schweiz zuzulassen. https://t.co/tQ3lskXh2Z
0
2
7
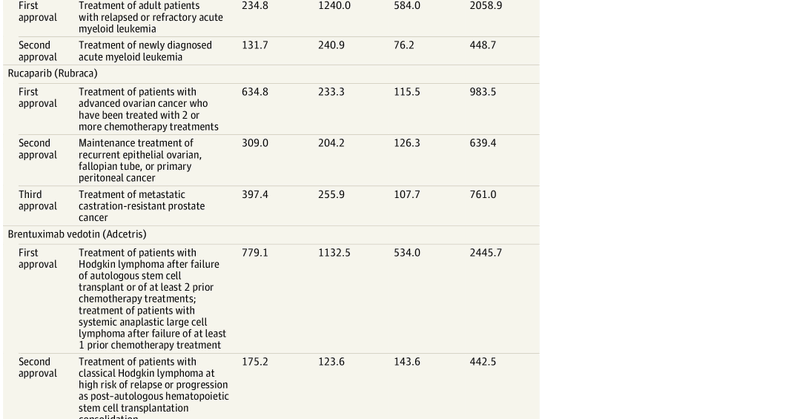
New piece: Drugs increasingly approved for many indications. Findings indicate substantially lower R&D costs for supplemental indications compared to first indications. More public data necessary to validate findings. with @ojwouters @LSEHealthPolicy
https://t.co/gEslklVwvN
jamanetwork.com
This Viewpoint compares the research and development costs for a drug’s first indication with supplemental indications to demonstrate that the cost of approval for supplemental indications may be...
0
9
30
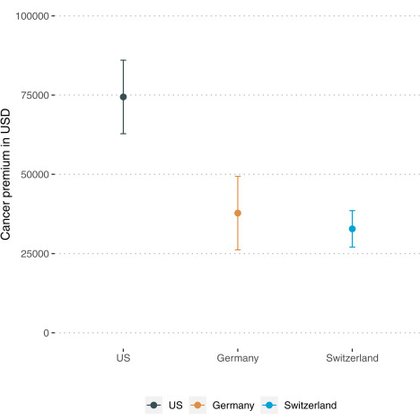
Cancer premium: Cancer drugs 3x more expensive than non-cancer drugs after adjusting for efficacy, prevalence, incidence & mortality. https://t.co/XLd5S0FsOR with @miquelsb2, @TheWonkologist Funding: @snsf_ch Covered by El Pais https://t.co/gvQjogRiNg & Swiss national news:
thelancet.com
Drug pricing reforms should target the cancer premium to improve access of patients to cancer drugs as well as to achieve equity across the different therapeutic areas and sustainability in the...
#Krebsmedikamente sind dreimal teurer als andere Medikamente: Das zeigt eine neue Studie. Eine medizinische Erklärung für den Preisunterschied konnten die Forscherinnen und Forscher nicht finden.
0
10
27