Olivier Wouters
@ojwouters
Followers
512
Following
576
Media
4
Statuses
164
Associate Professor @BrownHSPP Affiliate Faculty @PORTAL_Research Investigating global disparities in access to medicines
London, England
Joined June 2015
In our new @NEJM piece, we explore how the US came to spend billions of $$ over the past decade on a medicine first approved more than 40 years ago @wbfeldman has put together a nice summary of our proposed solutions @SeanTu2 @PORTAL_Research
https://t.co/mf19WsL2iV
nejm.org
Product hops to albuterol inhalers containing hydrofluoroalkane rather than chlorofluorocarbons cost payers and patients billions of dollars. Without patent and regulatory reform, this pattern is l...
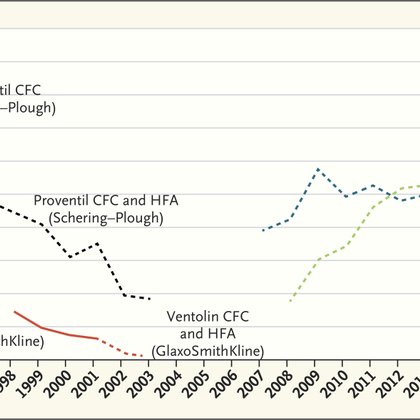
1/ In 2008, @US_FDA phased out #albuterol inhalers w/ ozone-depleting CFCs, including several generics. Since then, brand-name firms have reaped $14 billion in sales on albuterol products--though the active ingredient went off #patent in 1989! https://t.co/MrORdRNaZa 🧵👇
1
4
15
8/ Join Health Affairs’ Jessica Bylander (@jebylander) and me for a virtual “journal club” on October 21st at 11am EDT (4pm BST) where we’ll talk about the paper. Message me if you’d like to attend! https://t.co/TnTJaH6tIL
0
0
1
7/ Governments and companies urgently need to come up with strategies to address these disparities. Launch delays prevent large numbers of people from accessing potentially life-saving treatment, with severe consequences for health inequalities globally.
1
0
0
6/ We used the WHO list to select our sample because we wanted to identify drugs that are widely considered to be clinically important and for which timely global access is therefore desirable.
1
0
0
5/ Our analysis was based on data, sourced from IQVIA, on the timing of new drug launches in 90 countries from 1982 to 2024. We restricted our analysis to products on the WHO’s Essential Medicines List that came into medical use anywhere globally from 1982 onward.
1
0
2
4/ The bad news is that the gap between richer (high- and upper-middle-income) and poorer (lower-middle- and low-income) countries has remained relatively unchanged over time.
1
1
1
3/ The good news is that novel therapies are reaching countries at all income levels quicker today than they did back in the 1980s, 1990s, and 2000s.
1
0
0
2/ From the first launch globally, the median time to availability was under 3 yrs for high-income countries, compared to roughly 7 yrs for lower-middle-income countries and 8 yrs for low-income countries.
1
1
0
New study out in @Health_Affairs in which we analyzed how long it takes for new medicines to reach low- and middle-income countries. The bottom line: We observed vast inequities in global access to medicines. https://t.co/piudTABCrY
@LSEStatistics
healthaffairs.org
Little is known about how long it takes for new medicines to reach countries with different income levels. We analyzed data, sourced from IQVIA, on the timing of new drug launches in seventy-five...
2
3
6
Thanks to @ojwouters for visiting @TuftsCEVR and a truly excellent presentation on drug R&D expenditures, #HTA policies, & drug #patents. @Brown_SPH @PORTAL_Research @UW_Pharmacy @LSE @ihdezdelso
0
4
11
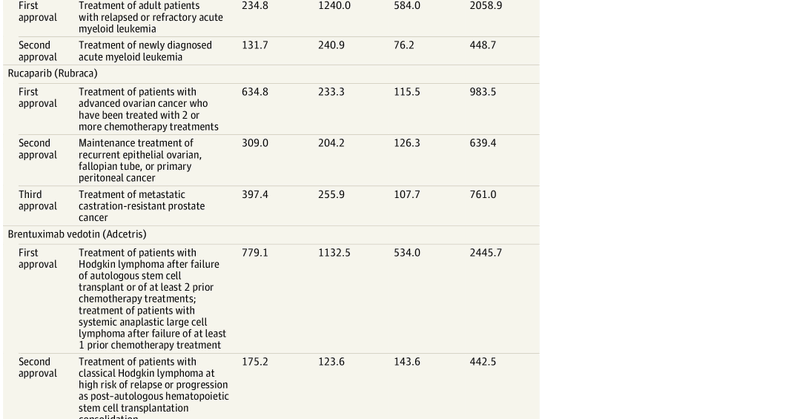
New piece: Drugs increasingly approved for many indications. Findings indicate substantially lower R&D costs for supplemental indications compared to first indications. More public data necessary to validate findings. with @ojwouters @LSEHealthPolicy
https://t.co/gEslklVwvN
jamanetwork.com
This Viewpoint compares the research and development costs for a drug’s first indication with supplemental indications to demonstrate that the cost of approval for supplemental indications may be...
0
9
30
🚨New @TheLancetOncol research was published right before #ASCO23 with a team of talented co-authors from around the 🌏, led by myself and @oncology_bg My post #ASCO2023 🧵👇 https://t.co/Jam0ubcBv0
3
9
33
In our new publication at Research Policy with @abanalestanol, @JofreBonet, Giulia Iori, Michele Tumminello and Pietro Vassallo, we evaluate the incremental impact of the Research Excellence Framework (REF) 2014 in the fields of Economics and Business.1/5
1
6
20
Regulated information sources for anticancer drugs in Europe fail to address the information needs of patients, finds study. If patients lack access to such information, clinical decisions may not align with their preferences and needs, warn researchers https://t.co/hkuUlUXyLV
bmj.com
Objective To evaluate the frequency with which relevant and accurate information about the benefits and related uncertainties of anticancer drugs are communicated to patients and clinicians in...
0
5
15
Want to learn more about pharmaceutical policy and economics? In a new report commissioned by @HealthFdn #REAL Centre, the Department’s Huseyin Naci & @robinjforrest_ explain pharmaceutical discovery, development, approval and adoption. ➡️ Access here: https://t.co/194uMOrvhc
0
15
29
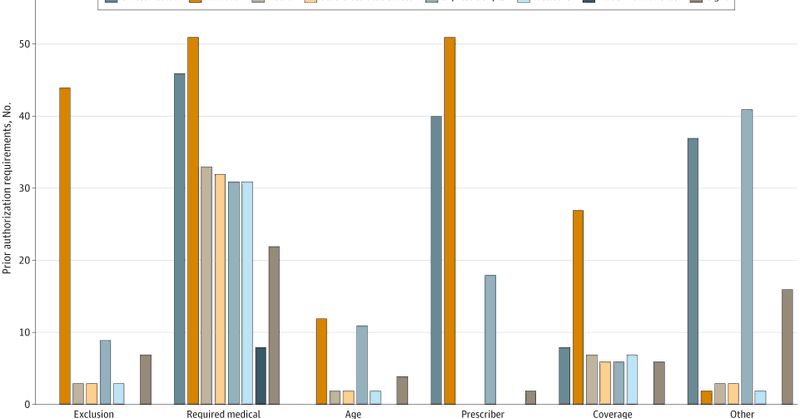
In new @JAMAHealthForum paper, @LSEHealthPolicy’s Huseyin Naci and @robinjforrest_ examine the insurer restrictions on new drugs in the US Medicare program➡️ https://t.co/gYNupVaVAm
@akesselheim @PORTAL_Research
jamanetwork.com
This cross-sectional study examines the characteristics of prior authorization policies for new drugs in Medicare Part D to understand whether they are consistent with US Food and Drug Administration...
1
5
11
Fascinating deep dive into the AstraZeneca-Fiocruz partnership that saw Brazil produce millions of doses of the AZ vaccine. Offers lots of insights into vaccine technology transfer @Emassard, Ken Shadlen (@LSE_ID) and Helena Moraes Achcar (@FGV_EAESP) https://t.co/GlNtDvhHMy
0
0
6
High drug prices are not justified by industry’s research and development spending, argue @Aris_Angelis and colleagues. From 1999 to 2018, the world’s 15 largest biopharmaceutical companies spent more on selling, general, and admin activities than on R&D https://t.co/VpJchNdK9y
bmj.com
Aris Angelis and colleagues argue that by refocusing their spending drug companies could provide more innovative drugs at affordable prices Concerns over the prices of new medicines have been growing...
3
56
58
Are high drug prices justified? In our @bmj_latest Analysis we investigate the spending of the 15 largest biopharmaceutical companies over 20 years and review available evidence on the added therapeutic benefit of new medicines. @ojwouters @ElsTorreele @mckee @LSHTM_GHECO
High drug prices are not justified by industry’s research and development spending, argue @Aris_Angelis and colleagues. From 1999 to 2018, the world’s 15 largest biopharmaceutical companies spent more on selling, general, and admin activities than on R&D https://t.co/VpJchNdK9y
1
17
33
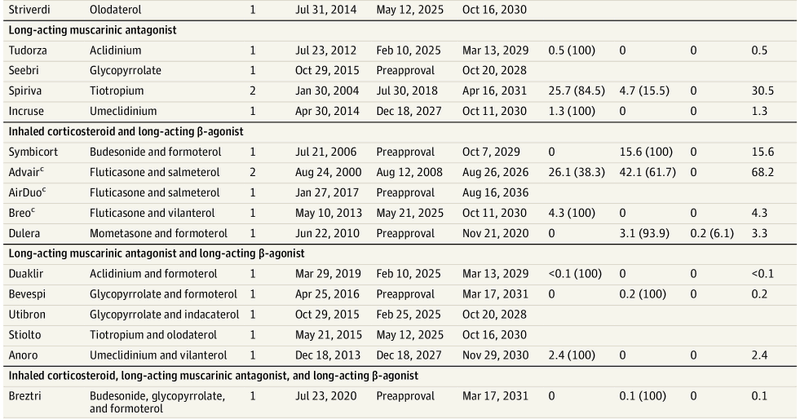
From 2000-2021, manufacturers earned $111 billion on brand-name #inhalers in the US AFTER patents on their active ingredients had expired. How? By patenting other aspects of the products (e.g., delivery devices). New piece out in @JAMA_current
https://t.co/2PQgsy4dAf 🧵
jamanetwork.com
This study quantifies the revenue earned on all brand-name inhalers approved by the US Food and Drug Administration from 2000 to 2021 and compared earnings before and after expiration of primary...
5
63
127
🚨New publication out in @LancetGH from us supporting increasingly complex decisions to list new cancer medicines on @WHO Essential Medicines List. 👇🏼 We shed light on processes, challenges, and a way forward #globaloncology
https://t.co/b4OIllAVNO
3
14
40