Qaiser Bashir
@DQBMD
Followers
2K
Following
961
Media
28
Statuses
753
Stem Cell Transplantation | Myeloma | Leukemia | Clinical Trials | Personal views | Educator | Mentor
Houston, TX
Joined May 2011
FDA grants accelerated approval to linvoseltamab-gcpt for relapsed or refractory multiple myeloma. @FDAOncology.
fda.gov
FDA grants accelerated approval to linvoseltamab-gcpt for relapsed or refractory multiple myeloma
0
3
6
RT @SocietyofHemOnc: ICYMI: FDA panel votes 6-2 in favor of daratumumab for high-risk smoldering multiple myeloma . .
0
3
0
#asco2025 Update: JNJ-5322 is a next-gen T-cell redirecting bispecific antibody targeting BCMA + GPRC5D with low-affinity CD3 for improved safety. SubQ dosing Q2W or Q4W. Step-up dose (5 mg) used to reduce CRS risk. #MultipleMyeloma #Immunotherapy
1
6
16
RT @MostafaFaisal14: How I approach hematopoietic stem cell transplantation for CML in a TKI world @BloodJournal.
ashpublications.org
Following the introduction of tyrosine kinase inhibitors (TKI), the number of patients undergoing allogeneic hematopoietic stem cell transplantation (allo-
0
14
0
RT @Nature: These are the 25 most cited papers published in the twenty-first century.
nature.com
Nature - A Nature analysis reveals the 25 highest-cited papers published this century and explores why they are breaking records.
0
108
0
RT @DrHKantarjian: Check out our comprehensive review on #AML from an @MDAndersonNews perspective, " Therapeutic horizon of acute myeloid l….
0
49
0
RT @Myeloma_Doc: #Myeloma Paper of the Day: CASSIOPEIA #MRD data show dara maintenance improved MRD-negative rates vs. observation regardle….
0
16
0
The BALANCE trial demonstrated that a 7-day antibiotic treatment strategy is non-inferior to a 14-day strategy for hospitalized patients with bloodstream infections. Limitation in context of HCT: Pts with severe immunosuppression were excluded
nejm.org
Bloodstream infections are associated with substantial morbidity and mortality. Early, appropriate antibiotic therapy is important, but the duration of treatment is uncertain. In a multicenter, non...
0
4
13
RT @TalhaBadarMD: Venetoclax and Decitabine vs Intensive Chemotherapy as Induction for Young Patients with Newly Diagnosed AML https://t.co….
0
61
0
RT @LeukDocJZ: Hot Off the Press 🚨🚨🚨ENHANCE-2- Randomized P3 Study of Aza/Magrolimab vs Physician Choice in Newly Dx TP53-mut AML- publishe….
ashpublications.org
Key PointsIn the ENHANCE-2 study, Magro/Aza did not improve OS of patients with TP53-mutated AML.Rates of grade ≥3 adverse events with Magro/Aza were compa
0
20
0
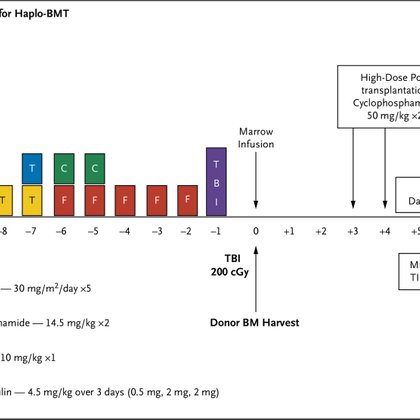
🚨 New study in NEJM Evidence: Haploidentical BMT with posttransplant cyclophosphamide in adults with sickle cell disease!.@BMTCTN @SCDAAorg @SCDFoundation @DrAdetolaKassim .
evidence.nejm.org
Related human leukocyte antigen (HLA)–haploidentical bone marrow transplantation (BMT) with posttransplant cyclophosphamide may be curative for sickle cell disease. However, graft failure, severe g...
1
0
2
RT @MM_Hub: ⭐ Multiple Myeloma spotlight: Latest survival and efficacy data from the phase III DREAMM-7 trial ⭐. During #ASH24, @dra_v_hung….
0
3
0