Alberto J Espay
@AlbertoEspay
Followers
9K
Following
13K
Media
617
Statuses
4K
#Neurology professor @uofcincy. I continue to try Bluesky since Nov 13, 2024 to foster a more collegial exchange of ideas. @albertoespay.bsky.social.
Cincinnati, OH
Joined October 2019
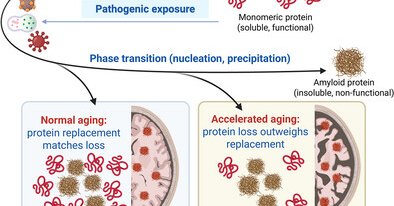
How does physics inform neurodegeneration? Supersaturation lowers the nucleation barrier for the precipitation of monomeric proteins into their pathological state. In normal aging, replacement matches loss; in accelerated aging, it does not. (1/4).
onlinelibrary.wiley.com
Soluble monomeric proteins precipitate via nucleation into insoluble amyloids in response to age-related exposures (e.g., microbes, nanoparticles). Persistent soluble-to-insoluble phase transition...
2
16
59
Here is a handful of thoughts on the idea of #Alzheimers being repackaged as an autoimmune disease. via @LinkedIn.
linkedin.com
For decades, the research pipeline for Alzheimer's and other neurodegenerative disorders has been built on the assumption that proteins must be the agents of disease, not the casualties. Because...
0
1
5
PS. Con my gratitud a los organizadores y líderes de la Asociación Colombiana de Neurología (@ACNeurologia) por todas las atenciones. Gracias Gabriel, Juan Diego, y el resto del buen equipo.
0
0
2
The XV Colombian Symposium of Movement Disorders just concluded in Santa Marta was nurturing to the soul. A vibrant, talented group of 175 seasoned clinicians and neurologists in training injected me with optimism. This is a premier @movedisorder meeting in South America.
5
7
39
In #Alzheimers news: “4 years on #lecanemab, the benefit tripled” … “3 years on #donanemab, the benefit doubled”, as summed by @Alzforum from #AAIC25 reported data. How the graphical illusion hides the acceleration of cognitive decline (9-part thread).
6
15
120
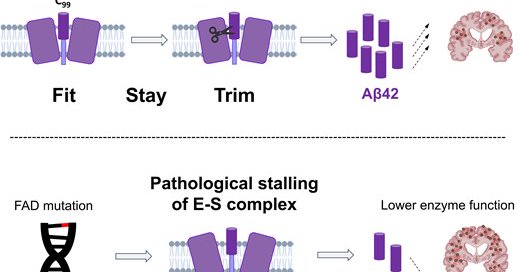
RT @ScienceofPD: "These findings suggest that restoring, not reducing, γ-secretase activity & monomeric Aβ42 levels above a compensation th….
academic.oup.com
Espay et al. challenge the view that Alzheimer’s disease is caused by increased gamma-secretase activity and overproduction of Aβ42. Instead, they suggest
0
1
0
RT @Brain1878: Espay et al. challenge the view that AD results from increased gamma-secretase activity and Aβ42 overproduction. Instead, th….
0
6
0
Amylyx halted the ORION trial in #PSP: no benefit from AMX0035 (sodium phenylbutyrate + taurursodiol). The question: a false negative trial (inadequate drug) or a true negative (protein aggregation + mitochondrial dysfunction not driving PSP)?.
investors.amylyx.com
- AMX0035 did not show differences compared to placebo on primary or secondary outcomes at Week 24 - AMX0035 continued to be generally well-tolerated CAMBRIDGE, Mass. --(BUSINESS WIRE)--Aug. 27,...
1
4
14
Plagiarism = copying words without credit. 'Idea plagiarism' = stealing insights without credit. With AI “remixing” text, sources get obscured, making idea theft easier. Copying words is bad, but copying findings or methods may be worse. Via @Nature .
nature.com
Nature - Researchers argue over whether ‘novel’ AI-generated works use others’ ideas without credit.
2
6
19