Aɴᴛᴏɴɪᴏ Pᴀssᴀʀᴏ
@APassaroMD
Followers
7K
Following
9K
Media
983
Statuses
6K
Med oncologist interested in lung cancer, biomarkers and drug development
Milan, Italy
Joined December 2016
That you are here - that life exists and identity,.That the powerful play goes on, and you may contribute a verse. Walt Whitman, Leaves of Grass (1892).
2
5
54
It was truly great to reconnect and discuss the latest in #lungcancer at this year’s CIOT in Venice. So many friends, global KOLs, and valuable time well spent advancing progress in lung cancer. #CIOT25
0
3
34
RT @Larvol: @ASCO 2025 Annual Meeting #LungCancer Recap - Here are the top trials from #ASCO25 with summary. Follow up for more oncology u….
0
8
0
Ph3 trials are always important, be careful not to issue guidance that would eliminate the only well‑established Ph3 data we have. Ph3 studies can change the whole perspective….▪️Chemo + IO: has never been a standard in this setting.▪️IMpower150: used here and there, but it.
#ASCO25 Update: Post-osimertinib progression in EGFRm NSCLC – how has the landscape evolved? (Pending approvals). 📍Pre-ASCO25 options.🔹 Chemo ± IO.🔹 Amivantamab regimens (MARIPOSA2).🔹 Atezoli+Bev+Pacli+Plat (IMpower150, ATTALAS).🔹 Local ablative therapy.🔹
0
0
10
#ASCO25 conc @JCO_ASCO 🏮.Zipalertinib in pts With EGFR ex20ins NSCLC Previously Treated With Platinum-B chemo With or Without Amivantamab .
ascopubs.org
PURPOSETo evaluate the safety and efficacy of zipalertinib, an irreversible epidermal growth factor receptor (EGFR) inhibitor, in pretreated patients with non–small cell lung cancer (NSCLC) harboring...
0
1
9
It might have seemed that all three treatment options achieved the same OS… and if that were the case, it could still be useful. But that’s not the case!! And today, fortunately for our patients, the Mariposa study has shown—for the first time in history—a significant OS.
Impactful slide highlighting the time AND financial burden of frontline treatment strategies for our patients with EGFR-positive NSCLC. How should this influence our treatment decisions? @JuliaRotow #ASCO25 @ASCO
0
3
11
Ph3 coming soon….😉.
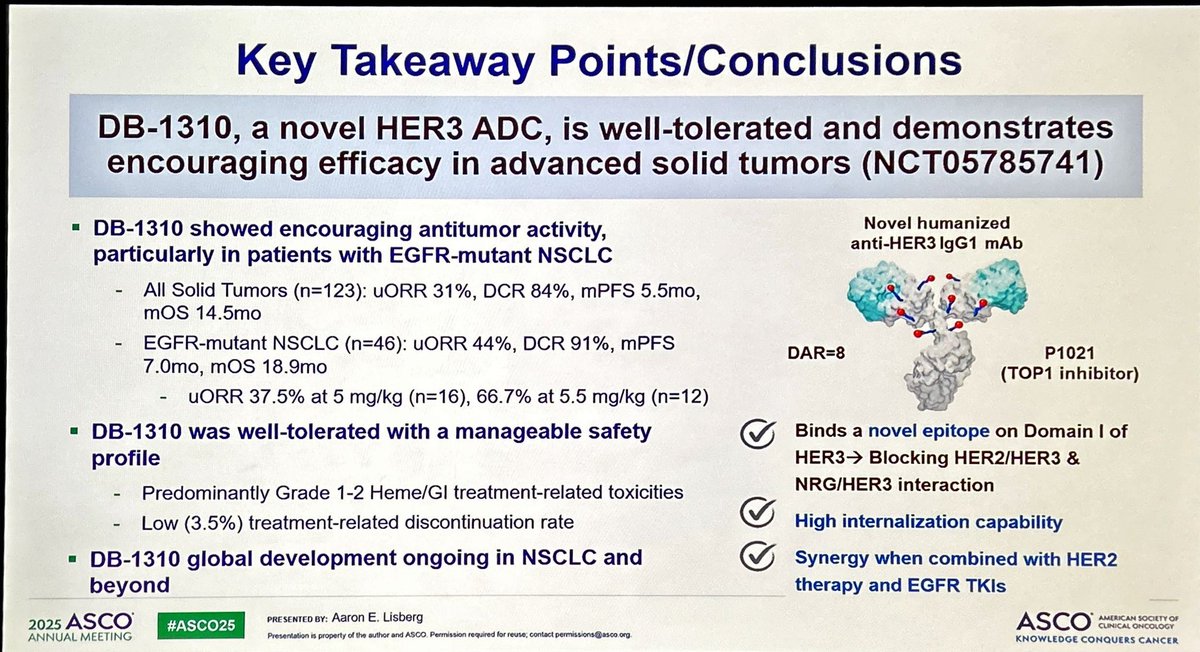
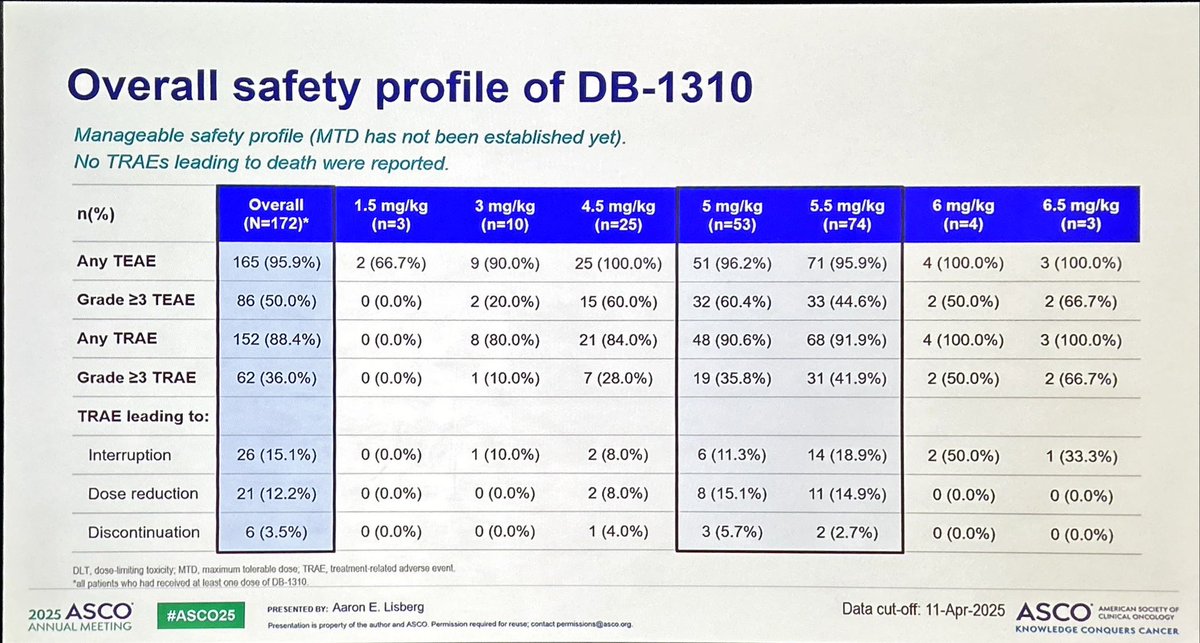
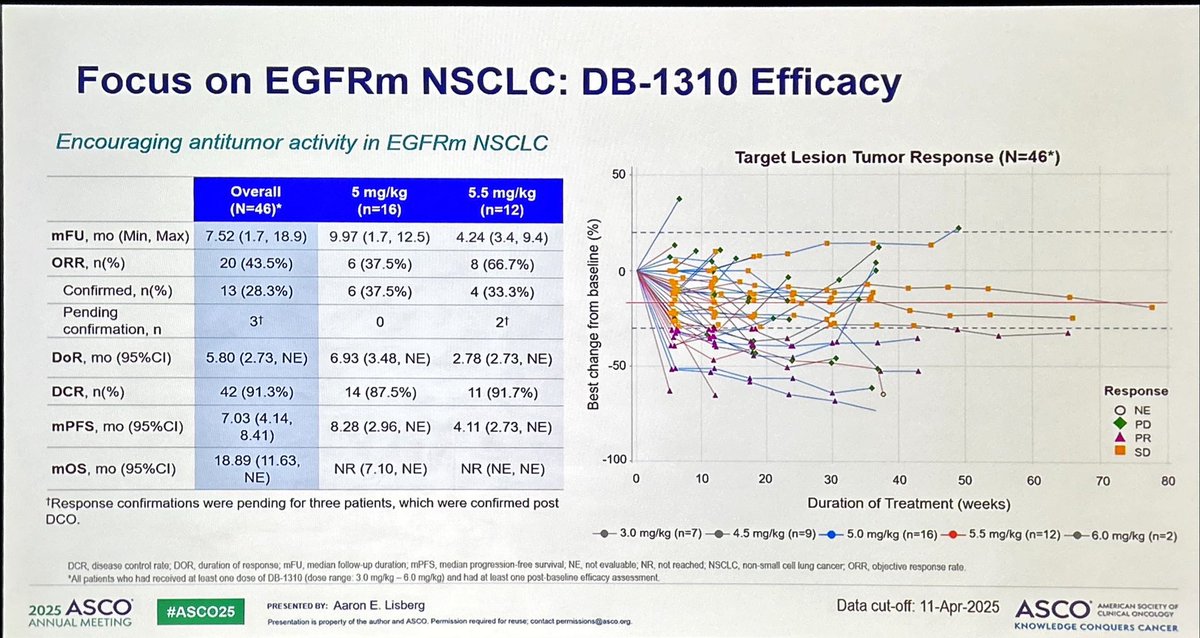
DB-1310 is a new ADC anti-HER3 with pantumor activity including NSCLC with promising activity in EGFRm 🫁. Based on disappointing results with HERTHENALung trial, next step should be DB-1310 + osimertinib in osimertinib-PD disease? #ASCO25
0
0
2
RT @Larvol: In 1L EGFR-sensitizing (Ex19del, L858R) NSCLC, treatment strategies have evolved from monotherapy TKIs—such as osimertinib (FLA….
0
25
0
🙌Congrats @Pignatelli_Lab & team for this amazing research effort, just out in @ScienceMagazine . A neuroimmune circuit mediates cancer cachexia-associated apathy
science.org
Cachexia, a severe wasting syndrome associated with inflammatory conditions, often leads to multiorgan failure and death. Patients with cachexia experience extreme fatigue, apathy, and clinical...
1
2
10
#ELCC25 ❤️🦋. After a great debate, it’s time to thank my amazing friends & colleagues Zofia and Myung-Ju for an outstanding session on EGFR—standing-room only, not a single seat left! .Grateful for such an engaging discussion.🙌
@APassaroMD is now debating on #EGFR and very clearly showing and stating that the time for combination is NOW. #ELCC25 @myESMO #ESMOambassadors
0
6
40
Don’t miss it!!! We are waiting for you 👇.
The upcoming #ELCC25 controversy session will feature perspectives from:.🗨️ Dr. Zofia Piotrowska of @MassGeneralNews - Osimertinib monotherapy remains our current standard of care.🗨️ @APassaroMD of @IEOufficiale - The time for TKI combinations is now. #lcsm #NSCLC #ELCC2025
0
1
7
🚨 Don’t miss it! 🚨.Join me, Zofia Piotrowska & Myung-Ju Ahn, tomorrow for a deep dive into this clinically hot topic, for patients with NSCLC harbouring EGFR mutations. Critical insights, real impact—be part of the conversation!.See you there! #ELCC25 💡
@OncBrothers @OncoAlert @ADesaiMD @FawziAbuRous @BrunaPellini @thenasheffect @drshieldsmd @StephenVLiu @RManochakian @Latinamd @LeXiuning @jillfeldman4 @EGFRResisters @EgfrUk Amivantamab’s biggest challenge has been its toxicity—the degree of OS benefit won’t translate in the real world without supportive measures. Kudos to the sponsor for investing in these (IMO) practice-changing supportive measures. BOOKMARK this slide! @OncoAlert #ELCC25
1
9
48
🔑 🇪🇺 #PressRelease .EMA CHMP recommends subcutaneous amivantamab (Q2W) for the treatment of patients with advanced EGFR-mutated NSCLC (MARIPOSA & PAPILLON)🪢.#LCSM .
jnj.com
Data from the Phase 3 PALOMA-3 study showed non-inferiority to intravenous administration meeting both co-primary pharmacokinetic (PK) endpoints, as well as a five-fold reduction in infusion-related...
1
16
56
In 2025, can we really justify a phase 3 trial for patients with oncogene-addicted #LungCancer without mandatory brain MRIs? .It’s time to prioritize patients over chasing poorly analyzed ‘significant’ advantages. We need a real paradigm shift❗️#LCSM.
2
2
38
COCOON study meets primary endpoint demonstrating statistically significant and clinically meaningful reduction in dermatologic reactions with easy-to-use prophylactic regimen for patients with EGFR-mutated NSCLC @EGFRResisters @EgfrUk @Exon20Group.
1
20
63
Subgroup analyses are crucial to full understand the benefits of new therapeutic standards. The FLAURA study, our current cornerstone, reported an OS HR of 0.80 in favor of Osi. This must serve as our foundation, despite the absolute survival benefit being less than 1year—a.
Need to see the HR and subgroups here, wish they hadn’t teased the “1-year” improvement in median OS. The interim OS HR was 0.8 and 5% diff at 2 years so curve probably very flat around the median meaning median diff can be huge with marginal HR. #LCSM.
0
2
6
We’ll see……….
Need to see the HR and subgroups here, wish they hadn’t teased the “1-year” improvement in median OS. The interim OS HR was 0.8 and 5% diff at 2 years so curve probably very flat around the median meaning median diff can be huge with marginal HR. #LCSM.
0
0
1
🚨Major breakthrough from #PressRelease. The MARIPOSA study reports a statistically significant and clinically meaningful improvement in overall survival (OS), reshaping the treatment landscape for patients with EGFR mutant NSCLC 🚀.
jnj.com
Median overall survival improvement expected to exceed one year First and only regimen with a survival benefit over current standard of care in first-line treatment of EGFR-mutated lung cancer
1
24
74
Very pleased this morning to meet @alshamsi2000 together with @curijoey during their visit to IEO. It’s an honor for me to call such expert a friend and to work together to support patients and advance cancer research through partnerships that go beyond borders 🇮🇹🙏🏻🇦🇪
0
3
25