John P. Richard
@jprichard53
Followers
16
Following
2
Media
0
Statuses
20
Joined March 2015
Just published in Biochemistry. A dynamic role for both hydride-donor and acceptor substrate fragments in driving an activating conformational change for glycerol phosphate dehydrogenase.
pubs.acs.org
Glycerol 3-phosphate dehydrogenase catalyzes reversible hydride transfer from glycerol 3-phosphate (G3P) to NAD+ to form dihydroxyacetone phosphate; from the truncated substrate ethylene glycol to...
1
0
1
Just published in Biochemistry. This paper highlights important catalytic advantages obtained by many enzymes that undergo substrate driven induced-fit conformational changes.
pubs.acs.org
Kinetic parameters are reported for glycerol 3-phosphate dehydrogenase (GPDH)-catalyzed hydride transfer from the whole substrate glycerol 3-phosphate (G3P) or truncated substrate ethylene glycol...
0
1
2
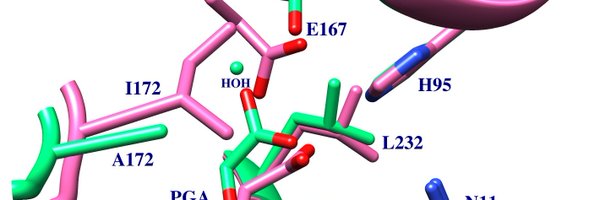
Read why the P168A and I172A substitutions at triosephosphate isomerase reduce the reactivity of a perfect enzyme to that for a small Bronsted base.
pubs.acs.org
The P168 and I172 side chains sit at the heart of the active site of triosephosphate isomerase (TIM) and play important roles in the catalysis of the isomerization reaction. The phosphodianion of...
0
1
3
Insights into Enzymatic Catalysis from Binding and Hydrolysis of Diadenosine Tetraphosphate by E. coli Adenylate Kinase | Biochemistry
pubs.acs.org
Adenylate kinases play a crucial role in cellular energy homeostasis through the interconversion of ATP, AMP, and ADP in all living organisms. Here, we explore how adenylate kinase (AdK) from...
0
0
0
Utilization of Cofactor Binding Energy for Enzyme Catalysis: Formate Dehydrogenase-Catalyzed Reactions of the Whole NAD Cofactor and Cofactor Pieces | Biochemistry
pubs.acs.org
The pressure to optimize enzymatic rate accelerations has driven the evolution of the induced-fit mechanism for enzyme catalysts where the binding interactions of nonreacting phosphodianion or...
0
0
2
Why are phosphate tags carried along with substrates through metabolic pathways? Read evidence for one model developed in studies on orotidine 5'-monophosphate decarboxylase.
pubs.acs.org
0
0
5
RT @BiochemistryACS: Cool enzyme alert: . Fernandez and Richard @UBuffalo expand the scope of adenylate kinase-catalyzed reactions by revea….
0
3
0
RT @LianetN: What a great tribute to Danny by Matsumura and Patrick, illustrating how his work paved the way to facilitate protein engineer….
0
3
0
The most proficient enzyme catalysts are activated by substrate-driven protein changes in conformation. Our Biochemistry paper discusses evidence that their appearance was a seminal event in enzyme evolution -
pubs.acs.org
Many enzymes that show a large specificity in binding the enzymatic transition state with a higher affinity than the substrate utilize substrate binding energy to drive protein conformational changes...
0
2
4
RT @J_A_C_S: Enzyme catalysts evolve and propagate effective structural motifs. Richard reports a tight cluster of ancient metabolic enzyme….
0
7
0
RT @SebVidalChem: Hey @J_A_C_S.Is it me or these two glucose-6-phosphate are missing their oxygen at position 6?. #vidalized .
0
1
0
RT @J_A_C_S: An extended Brønsted relationship for wild-type and mutant variants of triosephosphate isomerase reveals the role of key activ….
0
2
0
RT @J_A_C_S: Evolution of hydrophobic carboxylate binding pockets at decarboxylases is driven by stabilization of late enzymatic transition….
0
4
0
RT @ACSPublications: New from #ACR: Exploring the limits of the possible for enzymatic catalysis: .
0
4
0