The Liver Summit™ (TLS)
@TheLiverSummit
Followers
485
Following
818
Media
6
Statuses
68
CME & professional education on liver disease + its metabolic, GI, renal, cancer, etc. intersections. Expert-driven, live+hybrid. Flagship event: Sept 26, 2026.
United States
Joined July 2025
Where Discovery Meets Collaboration. A platform dedicated to advancing liver & cardiometabolic health through education, research, and global dialogue. Stay tuned for details on our inaugural meeting in 2026.
0
1
4
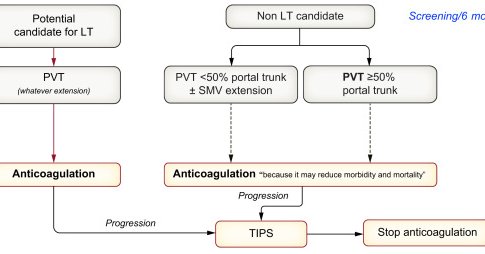
New @EASLnews guidelines on vascular liver diseases: from PVT to Budd–Chiari, PSVD, SOS, splanchnic aneurysms & hepatic AV fistulas. Updated info with evidence-graded, personalised recommendations and a clear push for multidisciplinary care @EASLedu
https://t.co/Uu9mOiUJn1
journal-of-hepatology.eu
Vascular diseases of the liver include portal vein thrombosis (with or without cirrhosis), portosinusoidal vascular disorder, Budd-Chiari syndrome, sinusoidal obstruction syndrome, non-obstructive...
0
20
72
True eradication of Hepatitis C will ultimately require an effective vaccine @HEP_Journal study showed conserved genotype 1–6 antigens & sequential 1a→3a immunogens generated broad, cross-reactive T-cell responses Important steps toward a HCV vaccine https://t.co/wFz9tVXsDC
0
1
5
Quarterback mentality on full display — 15 hours post-transplant and already lifting others up through organ-donation advocacy. Elite resilience. Proud to share your message. Best of luck in your recovery! 💪 💪 💚 #DonateLife
15 hours post–liver transplant and I’m already ready to attack the day. Feeling blessed, humbled, and beyond grateful for all your thoughts and prayers. U Matter #renewingathleteslives #nfl #shopkosar
0
1
3
Safety and efficacy of weekly pemvidutide versus placebo for metabolic dysfunction-associated steatohepatitis (IMPACT): 24-week results from a multicentre, randomised, double-blind, phase 2b study - @TheLancet #livertwitter
Safety and efficacy of weekly pemvidutide versus placebo for metabolic dysfunction-associated steatohepatitis (IMPACT): 24-week results from a multicentre, randomised, double-blind, phase 2b study - The Lancet
0
6
24
Original Article Determining safe washout period for immune checkpoint inhibitors prior to liver transplantation: An international retrospective cohort study Moeckli et al. #LiverX
https://t.co/CRO0CFqLHP
1
17
55
Novel MR biomarkers in MASH Multiparametric MRI (cT1, PDFF) correlated well with histologic response in MAESTRO-NASH Phase 3 trial of resmetirom Strengthens case for MR-based biomarkers as emerging tools for treatment response in MASH, both in trials & evolving clinical care
0
3
17
Wonderful case discussion on the intersection of obesity, AUD, and bariatric surgery by @BrighamWomens GI/Hepatology fellow @brittybb @AASLDtweets #TLM25
1
6
43
Great work from the Danish 🇩🇰 group — PEth predicts short-term hepatic decompensation independently of liver fibrosis markers, though its prognostic power fades over time. Important insights for how we interpret alcohol biomarkers clinically. #AASLD2025 #TLM2025
1
1
8
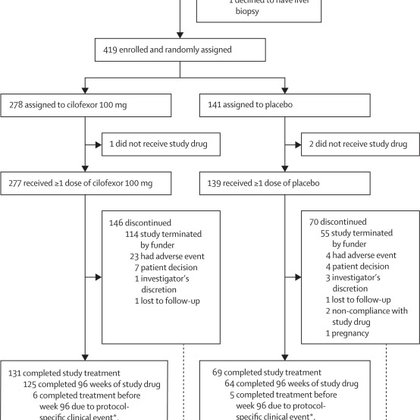
🧬The quest goes on for effective PSC therapy Phase 3 PRIMIS trial: FXR agonist cilofexor showed no antifibrotic benefit vs placebo at 96 weeks (fibrosis progression 31% vs 33%). Pruritus: 49% vs 36%; no new safety signals. #PSC #ClinicalTrials #LiverX
https://t.co/FyuGxPni5D
thelancet.com
Cilofexor did not significantly reduce the rate of fibrosis progression (vs placebo) in participants with non-cirrhotic PSC. A greater percentage of cilofexor-treated participants had pruritus than...
0
4
4
RASP-modulating therapy targets reactive aldehyde species—toxic lipid-derived molecules that drive oxidative stress and inflammation. By neutralizing these RASPs, it aims to reduce tissue injury and immune activation across inflammatory diseases, including liver injury.
0
2
2
Aldeyra reports positive Phase 2 proof-of-concept data in alcohol-associated hepatitis with RASP-modulating therapy—but won’t pursue AH, shifting focus to next-gen anti-inflammatory programs Encouraging signal, yet underscores the ongoing vacuum of industry commitment in AH
1
3
3
Serum bile acids within 1 yr post Kasai strongly predicted portal HTN & high-risk varices within 5 yrs (AUC 0.89–0.93) Early sBA monitoring may identify biliary atresia patients at risk for progressive portal complications despite good bilirubin clearance https://t.co/WGedlDu3j1
0
3
9
Glycine supplementation in severe obesity (100 mg/kg × 2 wks) ➜ ↑plasma glycine, improved 1-C metabolites and ↓triglycerides/aminotransferases — improved the glycine-serine-glutamate index Proof-of-concept for MASLD? @NaturePortfolio @SciReports
https://t.co/2WiBMG01GV
nature.com
Scientific Reports - Metabolic impact of dietary glycine supplementation in individuals with severe obesity
0
2
3
Dynamic weight trajectories best predict MASLD prognosis @HEP_Journal @AASLDtweets 📉 >5% loss→ improved liver stiffness 📈 >5% gain→ ↑ risk of liver events (aHR 1.84) & stiffness progression Obesity reversal restores outcomes similar to non-obese https://t.co/S3P0l9yLJs
journals.lww.com
and Results: By enrolling adult MASLD individuals with ≥2 weight measurements from 16 tertiary referral centers, we assessed how longitude weight change, including the following categories (stable...
0
3
7
Rivus Pharma oral metabolic accelerator HU6 reduced liver fat & improved adiposity markers in adults with MASH (Phase 2 M-ACCEL) Late-breaker @AASLDtweets #TLM2026 An oral therapy that improves both hepatic and systemic metabolism #MASH #MASLD #MetabolicHealth #Obesity
0
2
5
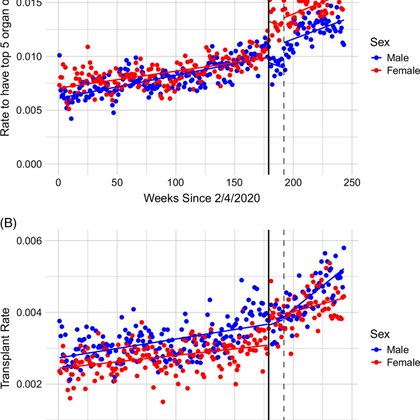
Early MELD 3.0 narrowed sex gaps in liver transplant access, but benefit faded—HEIGHT remains a key mediator @AASLDtweets @HEP_Journal Body size has always influenced allocation, is it time to refine how we account for it? #LiverX #LiverTransplant 🔗
journals.lww.com
transplant. Using a difference-in-differences design, we assessed the impact of MELD3.0 during immediate and 3-month postimplementation (“burn-in”) periods on receipt of (1) a top 5 organ offer or...
0
2
9
1st successful genetically engineered pig-to-human auxiliary liver xenotransplantation @JHepatology 10-gene edited pig liver sustained human function for 38 days (limited by thrombotic microangiopathy)—demonstrating feasibility as a bridge-to-transplant https://t.co/sZiw5ZMTZs
0
7
16
Novo Nordisk to acquire Akero Therapeutics, adding the FGF21 analog efruxifermin to its metabolic & liver-disease pipeline — underscoring growing industry confidence in FGF21-based therapies for MASH after Phase 2b data showed fibrosis improvement in F2/F3. Phase 3 on the horizon
1
2
3