Ofial_Lab
@OfialLab
Followers
313
Following
265
Media
6
Statuses
66
Developing nucleophilicity and electrophilicity scales at LMU München. We make colours disappear! PI run page. (inactive)
LMU München
Joined June 2021
Defining the Synthetic Scope of ortho-Quinone Methides by Quantifying their Electrophilicity (Armin R. Ofial @OfialLab and co-workers) #openaccess 🔓
chemistry-europe.onlinelibrary.wiley.com
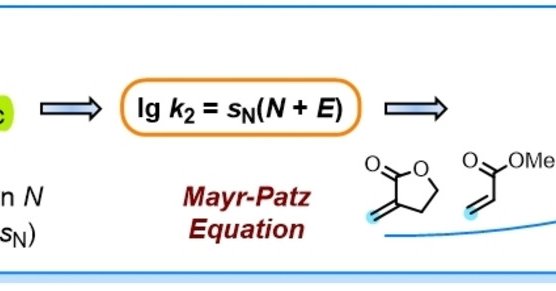
The electrophilicities E of ortho-quinone methides (oQMs) were determined by following the kinetics of oQM reactions with carbanions in DMSO. Embedding the oQMs in Mayr's reactivity scales facilita...
0
3
10
It was a real pleasure to give a lecture at the "Organisch-Chemischen Kolloquium" today at LMU Munich University @LMU_Muenchen. Thanks @SaitoGroup for the invitation and wonderful discussions with @OfialLab and all researchers from the chemistry department.
0
3
36
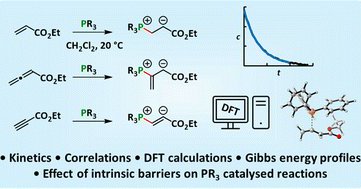
Reactivities of tertiary phosphines towards allenic, acetylenic, and vinylic Michael acceptors - now published in Chemical Science
pubs.rsc.org
The addition of phosphines (PR3) to Michael acceptors is a key step in many Lewis-base catalysed reactions. The kinetics of the reactions of ten phosphines with ethyl acrylate, ethyl allenoate, ethyl...
0
4
38
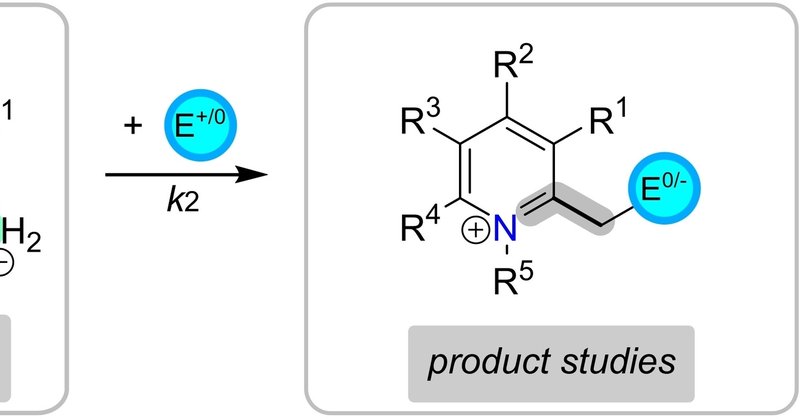
#EurJOC 2-Methylene-1,2-dihydropyridines (2-pyNHOs): Highly Nucleophilic Enamines by @MMHansmann_chem, @Ofial_Lab, and co-workers #OpenAccess
chemistry-europe.onlinelibrary.wiley.com
2-Methylene-1,2-dihydropyridines (2-pyNHOs) are enamines with strongly polarized exocyclic π-bond. The 2-pyNHOs react with quinone methides to give zwitterionic adducts or the entirely neutral...
0
2
6
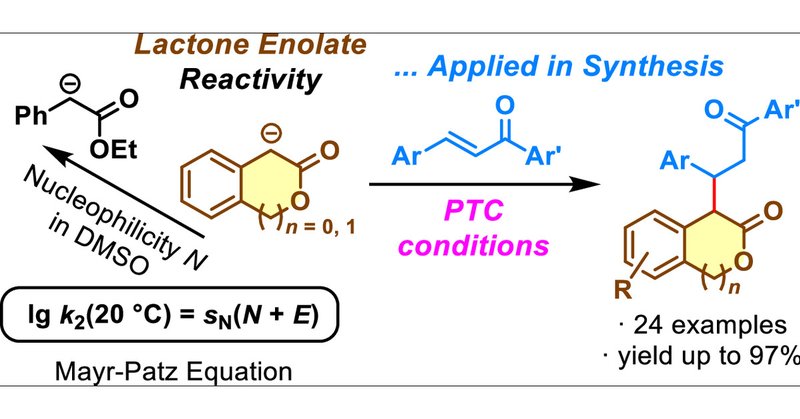
Happy to see results from the cooperation with the group of Antonio Massa (Univ. Salerno) published in @JOC_OL ! https://t.co/5Frt3JRN2v
pubs.acs.org
Owing to stereoelectronic effects, lactones often deviate in reactivity from their open-chain ester analogues as demonstrated by the CH acidity (in DMSO) of 3-isochromanone (pKa = 18.8) and 2-couma...
0
2
29
Reactivity of Electrophilic Trifluoromethylating Reagents by Armin R. Ofial, Herbert MayrH, and co-workers (@OfialLab, @Mayrs_Scale, @LMU_Muenchen)
1
6
34
Finish of the year: A new class of mesoionic NHOs just accepted @angew_chem. Big thanks to the group as well as the joint work with @OfialLab and @RJ_Mayer. Happy New Year! https://t.co/bLSkzRB7Mo
3
12
122
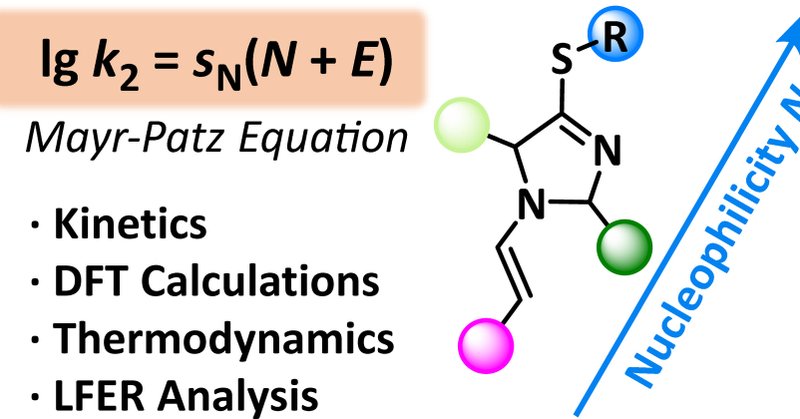
Nucleophilicity of 4-(Alkylthio)-3-imidazoline Derived Enamines (Ofial, Trapp, et al.) @TrappLab, @OfialLab
chemistry-europe.onlinelibrary.wiley.com
Enamine activation is a key method to enhance the reactivity of carbonyl compounds in α-alkylation reactions. In spite of their possible role in prebiotic chemistry, enamines derived from aldehydes...
0
4
16
Check out our reactivity database! It is a compilation of electrophilicity and nucleophilicity parameters for a wide range of compounds. https://t.co/dqpyylIhxT
0
2
11
Armin R. Ofial (@OfialLab) of @LMU_Muenchen presents a contributed talk @ESOR2023 on the upper limit of nucleophilicity scales: mesoionic N-heterocyclic olefins. #ESOR2023
0
8
25
We invite you to join our Virtual Symposium on October 11, moderated by @GrebGroup, in which four experts will present and discuss exciting aspects of modern Lewis-acid Chemistry. Do not miss this opportunity, registration is open! 👇 https://t.co/z1Zuo2ZXH2
0
31
69
Our joint work with @OfialLab is just accepted @angew_chem. Big thanks to the team for their great effort! https://t.co/glkMzIk3VE
3
9
94
Great to see Magenta's @MSCActions "SImCat" work - The effect of S-alkylation on organocatalytic enamine activation through imidazolidine-4-thiones - in cooperation with @TrappLab now published in ChemComm -
pubs.rsc.org
Imidazolidine-4-thiones have been suggested as potential prebiotic organocatalysts for light-driven α-alkylations of aldehydes by bromoacetonitrile. However, imidazolidine-4-thiones react with...
0
1
6
Hammond's Postulate - some new insights on a classic of physical organic chemistry ( https://t.co/Evyosi1ISS)
0
9
51
Happy to see our ICPOC25 Hiroshima conference paper "Reactivity of electrophilic cyclopropanes" with nice contributions by Andreas published! Read on https://t.co/xcP8Ghcodw
@degruyter_pub
degruyterbrill.com
Cyclopropanes that carry an electron-accepting group react as electrophiles in polar, ring-opening reactions. Analogous reactions at cyclopropanes with additional C2 substituents allow one to access...
0
0
7
Can Mayr’s reactivity scales be applied to rationalize the mechanism of 3+2 cycloadditions? Absolutely! Check out Le‘s massive kinetic work in @J_A_C_S to which I contributed the computational analysis. @OfialLab
https://t.co/w36IttMPoo
0
9
42
Check out our recent article live now in @J_A_C_S! Big congratulations to Le Li who obtained the interesting kinetic data and @RJ_Mayer who contributed the computational analysis!! Excellent work! https://t.co/l2qj5gRPpI
pubs.acs.org
Diazoalkanes are ambiphilic 1,3-dipoles that undergo fast Huisgen cycloadditions with both electron-rich and electron-poor dipolarophiles but react slowly with alkenes of low polarity. Frontier...
0
2
22
📢Together with our Board Member @samilakhdar1, we are proud to present the upcoming talk by Prof. Scott Denmark as part of the Physical Organic Chemistry webinar series! Save the date and join us this Thursday at 4 pm CET 👉 https://t.co/dUFqRPMqso
1
28
80
Nucleophilicities of Cyclic alpha-Diazo Carbonyl Compounds by Armin R. Ofial and co-workers (@OfialLab @LMU_Muenchen) #OpenAccess
https://t.co/rT7Sco2J30
0
4
25