Leonori Lab
@LeonoriLab
Followers
10K
Following
2K
Media
8
Statuses
245
Group-run account for the Leonori Research Group at RWTH Aachen University. Catalysis. Photoredox. Organic Synthesis.
Aachen, Germany
Joined September 2017
RT @alexandros4285: 🎓A fully funded PhD position in PHOTOCATALYSIS is available in the Ruffoni Research Group at Kiel University. 📝 📩send:….
0
46
0
RT @n_yasukawa: Checkout the Front Cover Art for our manuscript: "Photocatalytic Strategy for Decyanative Transformations Enabled by Amine-….
0
3
0
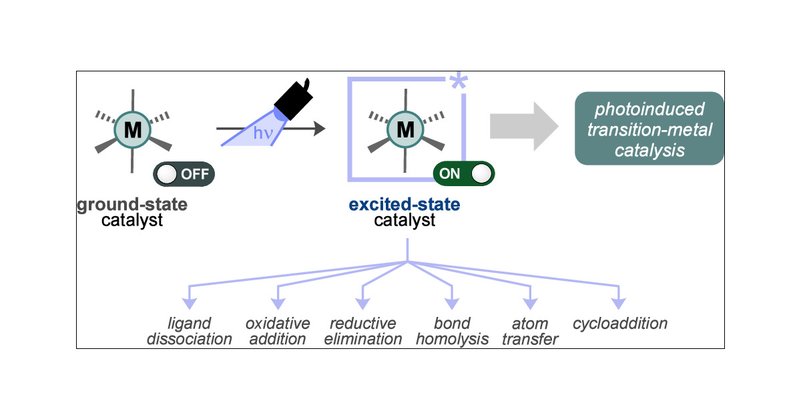
RT @FabioJulia7: If you wonder about how to harness innate excited-state reactivity in TM photocatalysis to design new synthetic methods, y….
pubs.acs.org
Transition metal catalysis is an indispensable tool for organic synthesis that has been harnessed, modulated, and perfected for many decades by careful selection of metal centers and ligands, giving...
0
23
0
Happy to share our latest results in @angew_chemie on the photochemical permutation of indazoles into benzimidazoles @RWTH .Also, make sure you check a related work from the @SarpongGroup also in @angew_chem .
3
8
119
RT @alexandros4285: Join our team! We are seeking passionate PDRA and PhD candidates interested in exploring synthetic methodologies and ph….
0
65
0
RT @synlett_journal: Discover the latest research of Hairong Lyu @HairongLyu and co-workers highlighting the use of a sp²-sp³ #diboron reag….
0
3
0
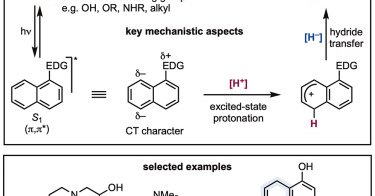
RT @Chem_CP: Online now: Excited-state protonation and reduction enables the umpolung Birch reduction of naphthalenes .
0
19
0
Congratulations to the authors @J_CorpasChem @evariverachao @dr_emarpa @alexandros4285 and the rest of the team including our academic partners and industrial collaborators in Minakem!.
0
0
3
Our latest publication in @Chem_CP demonstrates the umpolung Birch-like reduction of naphthalenes by photoexcitation employing a very mild acidic solvent (HFIP) and amine-boranes as reductants with high functional group compatibility ⬇️🔗.
cell.com
The Birch reaction is a key reaction for increasing sp3 content in organic molecules, but it traditionally requires strong reductants. This article presents a new strategy that reverses the nature of...
1
16
143
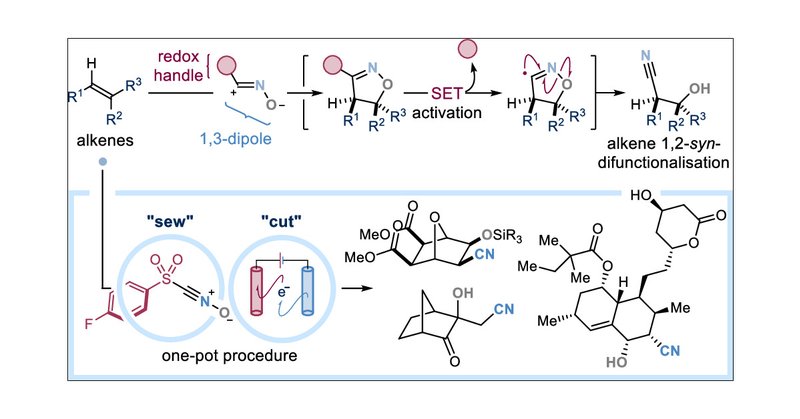
RT @GEMCrisenza: Thrilled to see our first contribution out in @J_A_C_S! @uaitaciano, Roberto, Órla & Ben developed a practical, broad-scop….
pubs.acs.org
Stereoselective alkene 1,2-difunctionalization is a privileged strategy to access three-dimensional C(sp3)-rich chiral molecules from readily available “flat” carbon feedstocks. State-of-the-art...
0
27
0
@Nature Well done to Baptiste, @MaialenAlonso8, @GiovanniLonardi, Dilara, Connie, @tds_fm, Yan and @alexandros4285. Thank you also to our collaborators Volker, Maria and Martin from @sanofi and Pep from @JanssenUS.
0
0
7
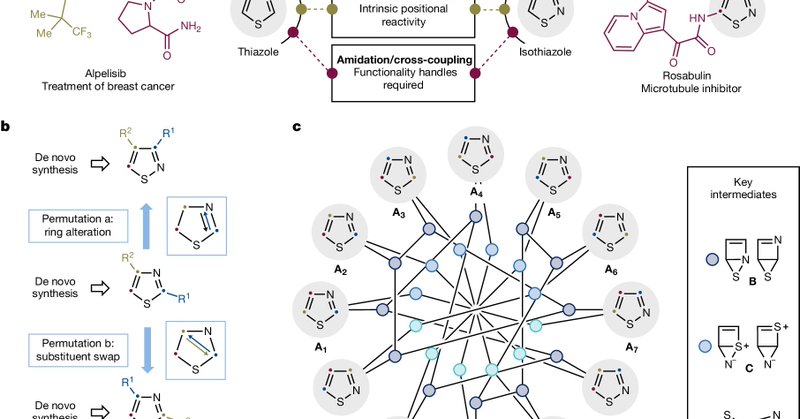
@Nature Building on pioneering studies by Pavlik, Maeda, and Vernin, this research demonstrates a way to reconfigure heteroaromatic structures—enabling one ring system to serve as a starting material for synthesizing another.
0
0
5
Excited to share our latest work on chemical permutation, now published in @Nature! Using photochemical conditions, we've developed a method to selectively and predictably transform the structures of thiazoles, isothiazoles, and other azole systems.
6
28
231
RT @J_CorpasChem: Check out our most recent work for the synthesis of alkynyl boranes from SOMOphilic alkynes as part of the special issue….
0
13
0