Zhen
@zhenliuchem
Followers
292
Following
297
Media
2
Statuses
161
Research group leader in the field of Biocatalysis
Beijing
Joined January 2019
We are excited to share our recent work on a biocatalytic atroposelective platform enabled by engineered desaturases: https://t.co/81qxCaqMIa MD-guided directed evolution played a key role in this effort. Congratulations to the whole team—more to come soon!
2
7
33
We would be glad to engage in further discussion if the reviewer has remaining questions.
0
0
0
Taken together–the above-mentioned points, along with the observed kinetic differences in mutagenesis experiments, the ability to undergo directed evolution, and inhibition by phenol additives–provide strong evidence that the reaction is enzyme-catalyzed.
1
0
0
While SDS can alter enzyme conformation, at low concentrations it does not necessarily cause complete denaturation. Our CD experiments further support this point.
1
0
0
SDS and other surfactants are commonly used as additives in enzymatic reactions (see for example: https://t.co/JeqoQALhvP;
https://t.co/c8TbCxF0ks;
https://t.co/ucjFs7mmYN)
1
0
0
First, the requirement for an enzyme can be readily tested through control experiments. Under standard conditions, reactions with FMN alone and FMN plus BSA produced no detectable anilines or phenols. This provides direct evidence that the engineered enzymes are essential.
1
0
0
One reviewer of our aniline paper suggested that the reaction is likely not enzymatic due to the addition of SDS, which is known to denature proteins at certain concentrations. We provide the following reasons to support that it is enzyme-catalyzed:
1
0
3
“I have four brothers and I’ve jumped into all sorts of things during my life. Learning new things has always been fun for me.” 2018 chemistry laureate @francesarnold received the #NobelPrize for her work using evolution to make better enzymes. Today she celebrates her birthday!
12
57
444
Really enjoying #Repartzymes Repurposed and Artificial Enzymes meeting organized by #TomWard of Uni. Basel. Many former group members are here! https://t.co/P3GoQqgUce
1
11
151
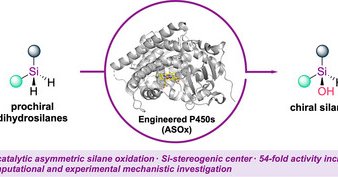
In our second paper, we demonstrate that chiral silanols containing Si-stereogenic centers can be prepared enzymatically with high efficiency and enantioselectivity. These structures were previously believed to be labile under aqueous conditions. https://t.co/MVMmeBrjOi
onlinelibrary.wiley.com
Engineered P450 enzymes were employed to catalyze the asymmetric oxidation of dihydrosilanes, producing Si-stereogenic chiral silanols with good yields and high enantioselectivies. The evolved...
0
1
6
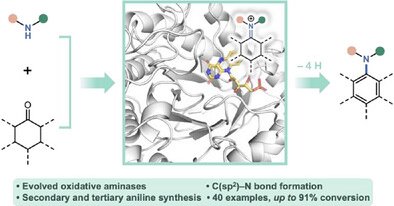
We have developed a biocatalytic system for synthesizing anilines through the enzymatic desaturation of cyclohexanone-derived imines:
onlinelibrary.wiley.com
A biocatalytic platform for aniline synthesis, based on the oxidative amination of readily available cyclohexanones, has been reported. Engineered variants exhibit broad substrate compatibility,...
1
1
5
Our team has recently published two research papers on Angewandte Chemie. Congrats to my students, Shuangyu Dai and Xiang Zhao, as well as our esteemed collaborators, Prof. Yunfang Yang and Dr. Xiahe Chen. The links are given below. Check these out if you are interested!
2
0
9
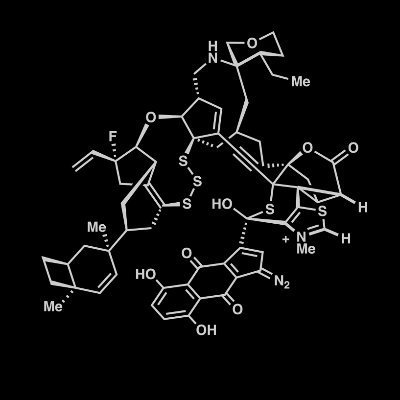
Thrilled to share our progress on the synthesis of highly oxidized DMOA-derived meroterpenoids—now out in JACS: https://t.co/fprfkoH4Sr! The unexpected reaction we stumbled upon in the final step turned out to be especially fascinating.
pubs.acs.org
The synthesis of complex natural products requires efficient control over chemoselectivity, stereoselectivity, and regioselectivity. Berkeleyacetals, a subfamily of 3,5-dimethylorsellinic acid...
#TotalSynthesis of DMOA-Derived Meroterpenoids: Achieving Selectivity in the Synthesis of (+)-Berkeleyacetal D and (+)-Peniciacetal I by Jianpeng Zhang, Xiaotong Luo, Jingfu Zhang, and Chao Li @LabChao in @J_A_C_S
https://t.co/a6WEMMZHSG
2
7
49
Now online: An Editorial highlighting some of the excellent covers featured in Nature Synthesis in 2024 as well as the research they represent. Thanks to our authors for submitting such great covers! https://t.co/oxg45LUkIN
0
2
8
Nov issue live🍂 Synthesis of chiral enones using flavoenzymes Fe-catalysed π-extension Cross-hydrodimerization of alkenes Ni-catalysed dicarbofunctionalization for polymers Insight into benzylidene-directed glycosylation (Continued) https://t.co/aLuE9xBi1j
1
3
9
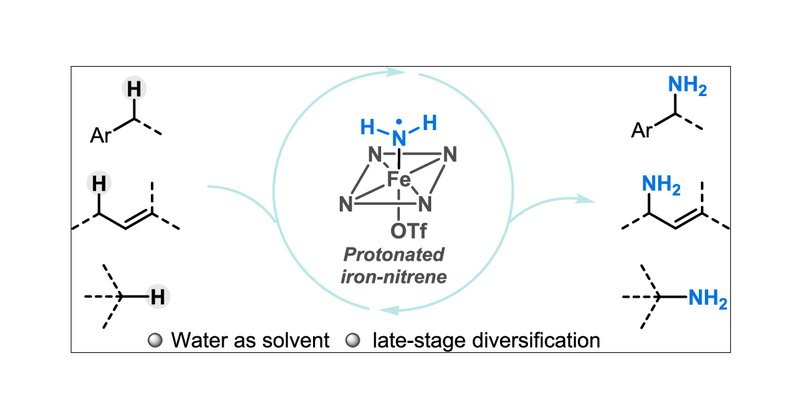
Inspired by the abiotic functions of cytochrome P450s, a chemocatalytic platform was developed to enable direct primary amination of various C(sp3)−H bonds under air and aqueous conditions.
pubs.acs.org
Primary amines are privileged molecules in drug development. Yet, there is a noticeable scarcity of methods for directly introducing a primary amine group into the ubiquitous C(sp3)–H bonds within...
2
1
14
Sequence-encoded monomers, a platform for translating difunctionalization into linear polymer backbones. A fun collaboration w/ @GuteGroup @pengliu_group & #BMSChemistry, appearing today in @NatureSynthesis. https://t.co/KdlmPFtR3K
0
6
101
Congratulations to Professor Frances Arnold (@francesarnold) on receiving the 2025 Priestley Medal! 🎉 The ACS is honoring Arnold, "for her pioneering contributions to the development of directed evolution as a method for chemical and biological design." https://t.co/DiVd74xDFU
cce.caltech.edu
Bestowing the chemical engineer with its highest honor, the American Chemical Society recognized Arnold's years of work developing and applying directed evolution to nature's catalysts.
4
15
90
Stereospecific Enzymatic Conversion of Boronic Acids to Amines by @DeirdreHanley4, Zi-Qi Li, @Shilong_Gao, Scott C. Virgil, @francesarnold at @CaltechCCE, and Edwin Alfonzo in @J_A_C_S
https://t.co/9RTW0zS0XN
0
24
119