The Otaka Lab.
@otaka_lab
Followers
61
Following
7
Media
31
Statuses
60
徳島大学薬学部・大学院薬科学教育部 機能分子合成薬学分野(大髙グループ)Otaka Lab. (Tokushima University)
Tokushima
Joined August 2021
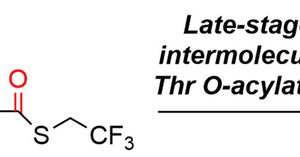
Check out our latest paper on @ChemEurJ.Late‐Stage Intermolecular O‐Peptidylation Protocol Enabled by Sequential Acyl Transfer on Thiol‐Incorporated Threonine Followed by Desulfurization - Sato - Chemistry – A European Journal - Wiley Online Library
chemistry-europe.onlinelibrary.wiley.com
The late-stage intermolecular O-acylation of the threonine (Thr) side chain was accomplished by reacting thiol-incorporated Thr with a peptide thioester in open air. The thioester, which contained...
0
2
11
RT @wakatepeptide57: 本日、第57回若手ペプチド夏の勉強会のホームページを公開しました。情報は、随時更新していきますので、ホームページおよびXをご確認下さい。.
sites.google.com
若手ペプチド夏の勉強会について
0
1
0
Professor Nitche @NitscheLab joined our research lab trip and we had a wonderful time exploring the scenic wonders of Shikoku!
0
1
11
RT @NitscheLab: Thank you very much Prof. Otaka and group for hosting me @TokushimaUniv and showing me the beautiful island of Shikoku. htt….
0
1
0
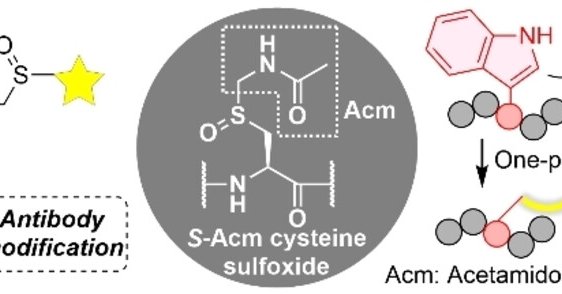
Check out our latest paper on @ChemEurope.Sulfoxide‐Mediated Cys‐Trp‐Selective Bioconjugation that Enables Protein Labeling and Peptide Heterodimerization - Kobayashi - ChemistryEurope - Wiley Online
chemistry-europe.onlinelibrary.wiley.com
A tryptophan selective modification was achieved that utilizes S-Acm cysteine sulfoxide under mildly acidic conditions in the presence of magnesium chloride. An optimum condition in an ionic liquid...
0
0
1
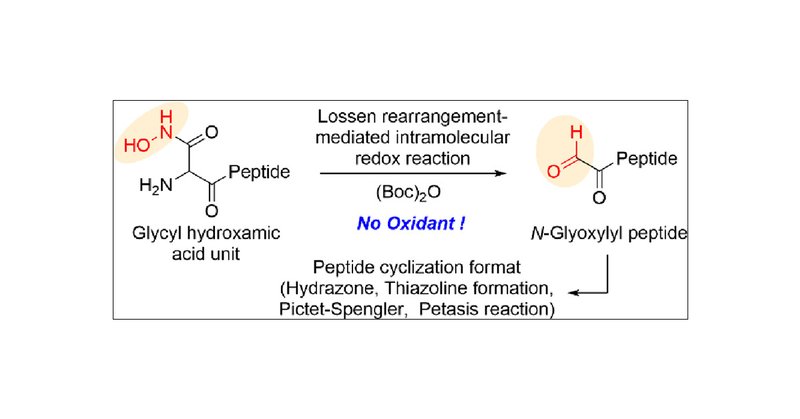
Check out our latest paper on @JOC_OL. Synthesis of N-Glyoxylyl Peptides Enabled by a Lossen Rearrangement-Induced Intramolecular Redox Reaction of N-Terminal Glycyl Hydroxamic Acid | Organic Letters
pubs.acs.org
An oxidant-free approach to the synthesis of N-glyoxylyl peptides has been developed that utilizes the Lossen rearrangement of the N-terminal glycyl hydroxamic acid residue. The synthesis proceeds...
0
2
11
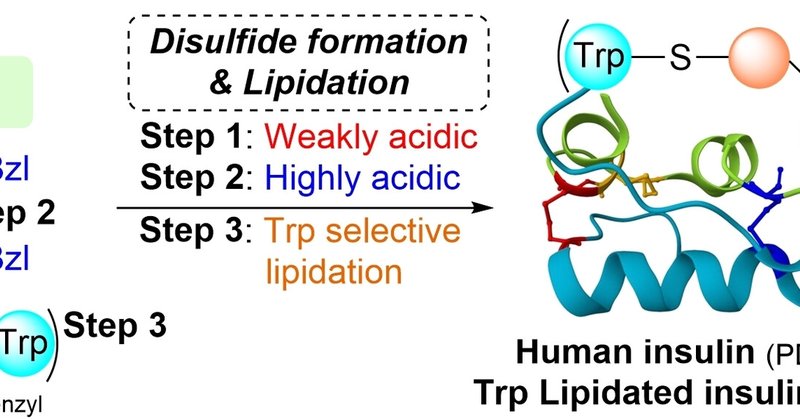
Check out our latest paper on @ChemEurope Advanced Insulin Synthesis by One‐pot/Stepwise Disulfide Bond Formation Enabled by S‐Protected Cysteine Sulfoxide - Hidaka - Chemistry – A European Journal - Wiley Online Library
chemistry-europe.onlinelibrary.wiley.com
The reaction of S-protected cysteine and the corresponding cysteine sulfoxide with increasing acidity of the reaction allowed one-pot/stepwise disulfide formation for insulin synthesis. The stepwise...
0
0
2
Commemorative photo of Professor Yasuhiro Kajihara and graduate students (Kobayashi, Hayashi, and Hidaka) who participated in IPS held in Brisbane. #AusPeptide2023
0
0
3
Commemorative photo of Professor Lara Malins and graduate students (Kobayashi, Hayashi, and Hidaka) who participated in IPS held in Brisbane. #AusPeptide2023
0
0
2
Commemorative photo of Professor Fernando Albericio and graduate students (Kobayashi, Hayashi, and Hidaka) who participated in IPS held in Brisbane. #AusPeptide2023
0
0
2