Ori Green
@origree1
Followers
204
Following
503
Media
0
Statuses
259
Joined May 2022
RT @morandilab: Many Congrats to @yannickbraegger,.@PaschkeAK, Nima Nasiri, @BenceBBotlik.and @Ffelician99 for this masterpiece! Oxidative….
science.org
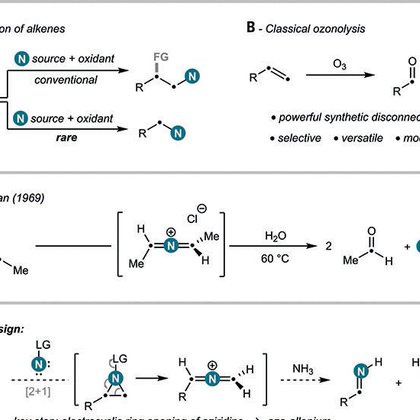
The synthesis of nitrogen-containing molecules through carbon–nitrogen (C–N) bond formation is critical for the discovery and preparation of medicines, agrochemicals, and materials. Here, we report...
0
50
0
RT @BismutoLab: Glad to see this out in @cenmag !! Many thanks Beth for the amazing highlight!.
cen.acs.org
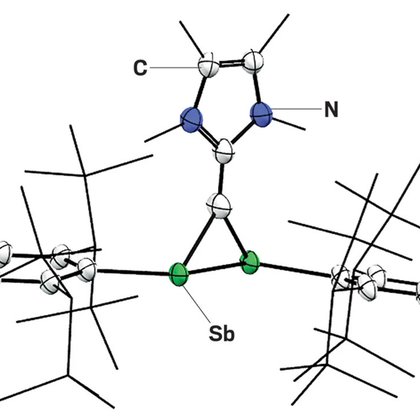
Chemists create heterocycles with multiple antimony atoms, including a stable, 3-membered ring
0
9
0
RT @ShabatGroup: The final version of this manuscript was published in @BioconjChem: Many thanks to @BaranLabReads….
pubs.acs.org
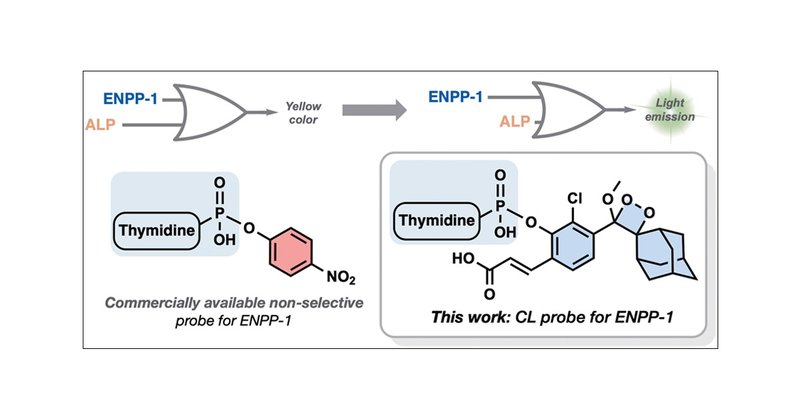
ENPP-1 is a transmembrane enzyme involved in nucleotide metabolism, and its overexpression is associated with various cancers, making it a potential therapeutic target and biomarker for early tumor...
0
7
0
RT @BismutoLab: A great Start of the year with the first @JACS of the group!! Synthesis of Heavy Inorganic Rings and their reactivity🧪 Con….
pubs.acs.org
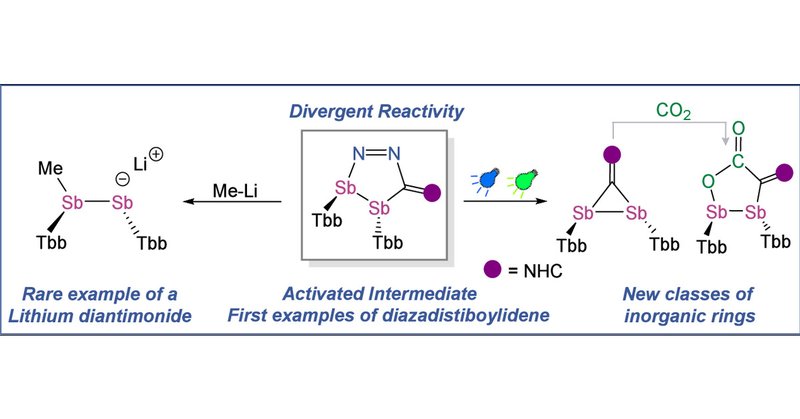
The chemistry of heterocycles containing “diaza” units has been extensively studied due to their applications ranging from pharmaceuticals to advanced materials. In contrast, heterocycles incorpora...
0
15
0
RT @BenhamouLab: Excited to share our latest publication in Heliyon! We developed fluorescent probes using RIBOTAC tech to visualize RNase….
0
6
0
RT @TimothyASu: POSTDOC AD:.I'm looking to hire a postdoc who can bring their expertise in low-valent main group molecular synthesis and ap….
0
58
0
RT @Aromaticist: 🥳Busy week for us here in @PoranneG! . 👩💻@awahab_pg's latest paper just hit the presses @PCCP! . 🤓Our adventures in the l….
0
2
0
RT @ShabatGroup: “Structure-Activity Optimization of Phenoxy-1,2-Dioxetane Precursors as Probes for Singlet Oxygen Yields Unprecedented Det….
0
15
0
RT @GroupMarek: Explore new frontiers in Organic Chemistry with our group's available PhD and postdoc positions! Motivated candidates, send….
0
44
0
RT @BenhamouLab: Our latest research is out in Macromolecular Bioscience! Congrats to Aseel & team on developing small molecule degraders t….
0
2
0
RT @sevenroediger: I am very happy that our study was published in Nature! Please check it out!.Thanks a lot to my co-authors @emilien_ls a….
0
6
0
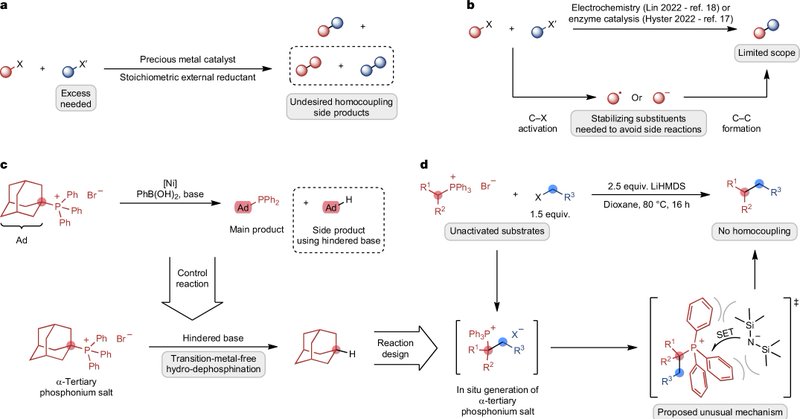
RT @morandilab: Many Congrats to @sevenroediger, Emilien Le Saux and Philip Böhm for uncovering a new mechanistic approach to alkyl-alkyl c….
nature.com
Nature - A transition-metal-free platform enables the formation of challenging C(sp3)–C(sp3) bonds in organic compounds via single-electron transfer, facilitating the coupling of...
0
35
0
RT @DobrovetskyG: It took us a few years and two papers to finally synthesize this P(III) cation. Fresh out of the oven: Sb‐to‐P Metathesi….
0
18
0
RT @AshrafBrik: Combing chemical protein synthesis and cell delivery reveals novel insights on the tail processing step of SUMO2 is now in….
0
8
0
RT @Aromaticist: Given the Xodus to 🦋, you can find me over there with the same handle: @Aromaticist. I'm still here for now, but I guess….
0
3
0
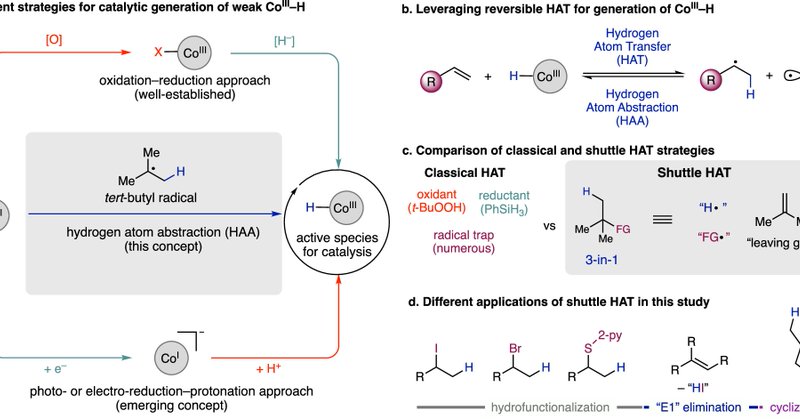
RT @W0TangClan: My first paper from the Morandi group. Shuttle catalysis greatly simplifies Co–H formation for MHAT reactivity – now all yo….
nature.com
Nature Communications - Hydrogen atom transfer (HAT) from a metal-hydride is a reliable and powerful method for functionalizing unsaturated C–C bonds in organic synthesis. Herein, the authors...
0
17
0
RT @morandilab: Congrats to Tanner @W0TangClan, Philip Blank, Andrea Brugnetti, Philip Boehm and Françoise Aouane - introducing the concep….
0
13
0
RT @morandilab: Congrats to @sevenroediger, Adriana Neves Vieira, Cosima Brudy, Mischa Trabesinger and Jan Hübscher - Olefination of Activa….
0
14
0
RT @chemosensors: 👉Imaging Tools for Chemical Biology .Edited by Lei Feng and Tony James (@chemosensors).🔗 🔍This bo….
books.rsc.org
This book provides a comprehensive overview of multimodal and combined techniques used for imaging applications in Chemical Biology, including afterglow lu
0
11
0