Alex Cusumano
@cusumano1_alex
Followers
95
Following
434
Media
0
Statuses
55
Caltech PhD candidate | Stoltz Group💡Pasadena, CA🌴NCSU ‘18
Joined August 2017
What a fantastic day to celebrate the launch of our new Integrative Sciences Building! Thrilled to be leading this effort with a team of world class faculty. @NCState_ISI @NCStateProvost
“A place where the sciences can come together and focus on the chemistry of life.” —Chancellor Woodson 🧬 Today marked the official groundbreaking of the Integrative Sciences Building — a space will that will unite fields focused on the world’s core building blocks: molecules.
0
5
40
Congratulations to Alex and Tianyi for their recent publication on the Origins of Enhanced Enantioselectivity in the Pd-Catalyzed Decarboxylative Allylic Alkylation of N-Benzoyl Lactams. Check it out here:
mdpi.com
We explore the origins of the marked improvement in enantioselectivity in the inner-sphere (PHOX)Pd-catalyzed allylic alkylation of N-benzoyl lactam nucleophiles over their carbocyclic counterparts....
0
3
23
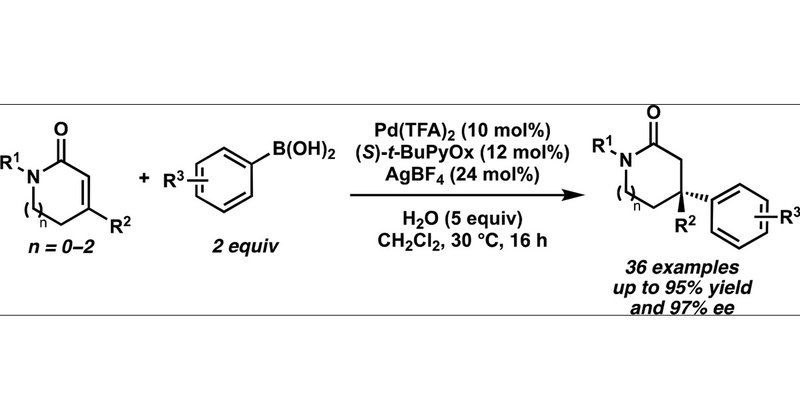
Congratulations to Tianyi, Yuji, and Alex for their recent publication on Palladium-Catalyzed Asymmetric Conjugate Addition of Arylboronic Acids to α,β-Unsaturated Lactams. Check it out here:
pubs.acs.org
Stereogenic nitrogen-containing heterocycles are ubiquitous in natural products and pharmaceutical compounds, but methods for their enantioselective construction have remained elusive. We report a...
0
13
95
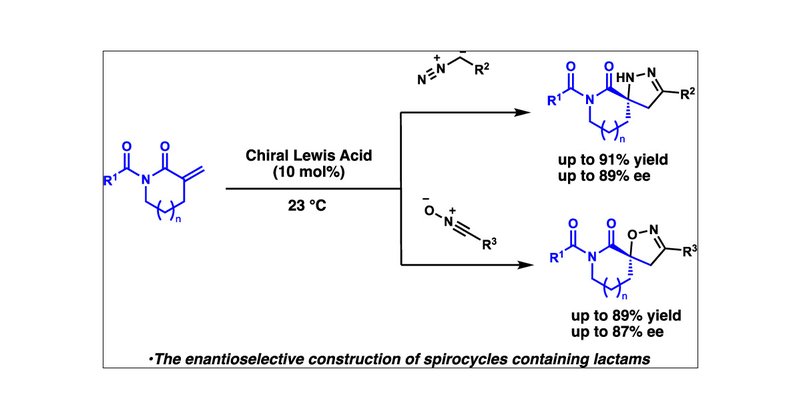
Congratulations to Yuji, Kevin, and Alex for their recent publication on Enantioselective 1,3-Dipolar Cycloadditions of α-Methylene Lactams to Construct Spirocycles. Check it out here: https://t.co/NofwyBX1SX
pubs.acs.org
Spirocyclic scaffolds are important motifs due to their potential to bestow favorable effects on pharmaceutical compounds. However, there is a need for efficient methods for their enantioselective...
1
9
82
Excited to finally share this project! A little bit of everything we love - Pd enolates, catalysis, phys org, theory, and a lot of stereocenters. We had an awesome team and a lot of fun! Stay tuned for more… 👀
Very exciting to see this dream of a project in press @J_A_C_S congrats @thestoltzgroup @CaltechCCE #proudadvisor Divergent Catalysis: Catalytic Asymmetric [4+2] Cycloaddition of Palladium Enolates
2
6
28
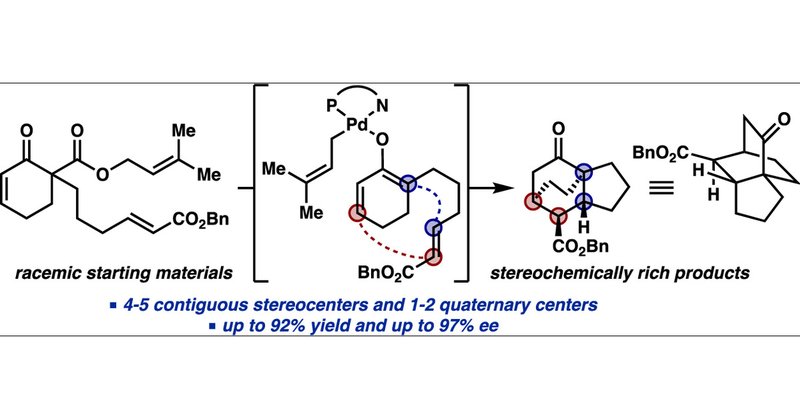
Very exciting to see this dream of a project in press @J_A_C_S congrats @thestoltzgroup @CaltechCCE #proudadvisor Divergent Catalysis: Catalytic Asymmetric [4+2] Cycloaddition of Palladium Enolates
pubs.acs.org
An asymmetric decarboxylative [4+2] cycloaddition from a catalytically generated chiral Pd enolate was developed, forging four contiguous stereocenters in a single transformation. This was achieved...
5
27
225
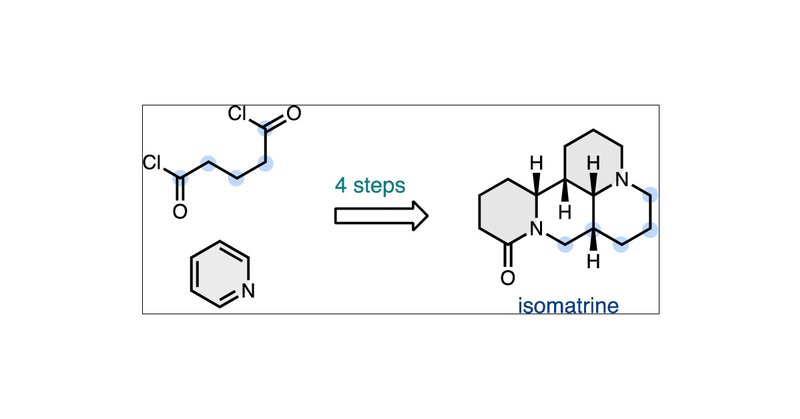
I'm soooo excited that it's finally live! A Pyridine Dearomatization Approach to the Matrine-Type Lupin Alkaloids! Thank you so much to everyone who made this project possible!! @sarah_reisman
https://t.co/5XONvJhjdp
pubs.acs.org
(+)-Matrine and (+)-isomatrine are tetracyclic alkaloids isolated from the plant Sophora flavescens, the roots of which are used in traditional Chinese medicine. Biosynthetically, these alkaloids are...
15
23
264
Beyond happy to share what will be the central focus of my PhD thesis. Thanks to @CAMalapit @NTaimoory @cbrig215 @Sanford_Lab for the hard work!
What's better than Halloween? NOTHING, but Pd-catalyzed decarbonylative fluoroalkylation is a close second 🎉🎃☠️🎃🎉 Congrats to the team! @LallooNaish @CAMalapit @NTaimoory @cbrig215
https://t.co/u8rtUzLT75
3
7
43
Check out our recent work discovering a reactive alpha-bromocaranone from (+)-carene that generates diverse chiral building blocks through radical or carbocation-mediated cyclopropyl fragmentation reactions! Great job Adrian and Zack!
0
4
43
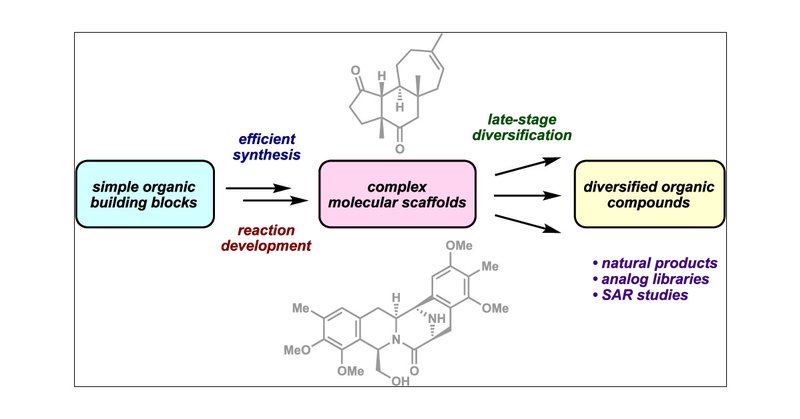
Late-Stage Diversification: A Motivating Force in Organic Synthesis @brianstoltz70 @thestoltzgroup @CaltechCCE @Caltech
pubs.acs.org
Interest in therapeutic discovery typically drives the preparation of natural product analogs, but these undertakings contribute significant advances for synthetic chemistry as well. The need for a...
0
10
47
Still miss those days! Eternally grateful for the mentorship @piercelab
@ianatonks @JenniferSchoma4 There is nothing better than mentoring undergrads to success though — right, @The_Boudreau (@PaulHergie), @cusumano1_alex (@brianstoltz70), @LallooNaish (@Sanford_Lab), @BenStemen (@uncchemistry), @CadeChem (@UWMadisonChem)? I hope colleagues embrace the joy of the experience!
0
0
2
Here, @thestoltzgroup presents a holistic mechanistic study of Ni-catalyzed enol ether formation, concluding that this reaction proceeds through C-O reductive elimination at nickel(II), an oxidation state of nickel that is much easier to access. https://t.co/yFtlysGl09
0
11
45
Check out the 1.4 release of Psi4! Release notes: https://t.co/qZvDF4vdjr Downloads: https://t.co/qZVaNxVisF
github.com
Advertised Version: 1.4 Continuous Version: 1.4 Release Date: 3 August 2021 Documentation: https://psicode.org/psi4manual/1.4.0/ Availability: Public, GitHub source, CMake build, Conda binary insta...
1
19
75
Congrats to Trevor and Alex on their latest research of Ni-catalyzed C-O bond formation through a proposed Ni(0)/Ni(II) redox cycle using dual experimental and computational studies!
pubs.acs.org
We present a series of experimental and computational mechanistic investigations of an unusually facile example of Ni-catalyzed C–O bond formation. Our method, originally reported in 2016, involves...
0
3
28
Check out our computational evaluation of our synthetic strategy toward ineleganolide using QM calculations, dedicated to Dale Boger for the Tetrahedron Prize 🎉Great work Alex @cusumano1_alex !!
0
3
32
Thanks again to my mentors and labmates! In honor of Prof. Dale Boger’s Tetrahedron Prize:
0
0
12
Congrats to Chris (@TrojanChemist), Fa, Kohei, Daisuke, and Katerina for their incredible contributions to the enantioselective syntheses of eburnamonine, eucophylline, & using a convergent coupling strategy to access epi-leucophyllidine!
1
5
54
And another one! Congrats @BHFrohock!
Congrats to @BHFrohock on his successful defense today - group PhD #12! Good luck in the Walczak group for your postdoc later this summer. @NCStateChem
0
0
1