Yuki Nagashima
@YukiNagash
Followers
314
Following
289
Media
1
Statuses
26
Asst. Prof./Univ of Tokyo (pharm)/JST-FOREST/Org. chem./Photochem./Main-group chem./ Comp. chem./Med. Chem./https://t.co/WX4303ZDhh
Tokyo
Joined November 2025
My representative works ACIE 2025 germylation https://t.co/9vIk0Q87z6 ACIE 2024 dearomatization https://t.co/SeV9PRrBsg Commun. Chem. 2024 VMP https://t.co/I1P28Ra3Qj Nat. Commun. 2023 silaboration https://t.co/LswtapJBkD Nat. Synth. 2023 Rh photocatalysis
onlinelibrary.wiley.com
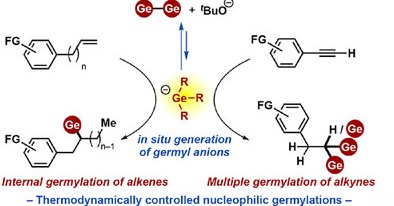
A thermodynamically controlled nucleophilic germylation of stable π bonds was achieved by germyl anion generated in situ via heterolytic cleavage of the Ge─Ge bond in the presence of KOtBu. This...
0
1
33
Our latest article regarding photosensitizer-free infrared-light-induced radical reactions is now available on @ChemRxiv ! Photosensitizer-free infrared-light-induced radical reactions https://t.co/6oHPq0XJ0Y
2
4
31
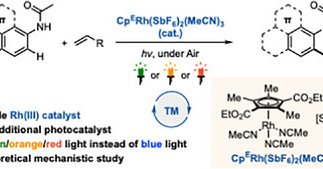
Our latest paper has been published in Chem. Eur. J. @ChemEurJ ! “Red and Orange‐Light‐Accelerated Rhodium‐Catalyzed Oxidative Olefination of Arenes” https://t.co/IEipauWYIk
chemistry-europe.onlinelibrary.wiley.com
Long-wavelength visible-light-accelerated Rh(III)-catalyzed olefination of arenes bearing anilides is reported. The method operates without an external photosensitizer or short-wavelength blue...
0
4
54
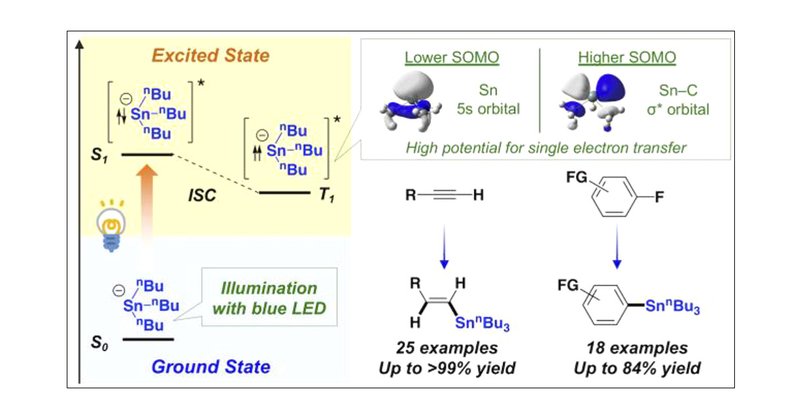
本研究は、以前のスズの光励起( https://t.co/YizK2COZbk)に関する研究を展開したものです。スズは重原子効果で三重項が効率よく利用できたのですが、ケイ素の場合はそうはいきません。そこで、ニッケルとの錯形成を利用し、ケイ素の励起状態を実現することで、新しいケイ素化反応が開発できました。
pubs.acs.org
We have developed photoboosted stannylation reactions of terminal alkynes (linear-selective hydrostannylation) and fluoroarenes (defluorostannylation), in which the stannyl anion is photoexcited to...
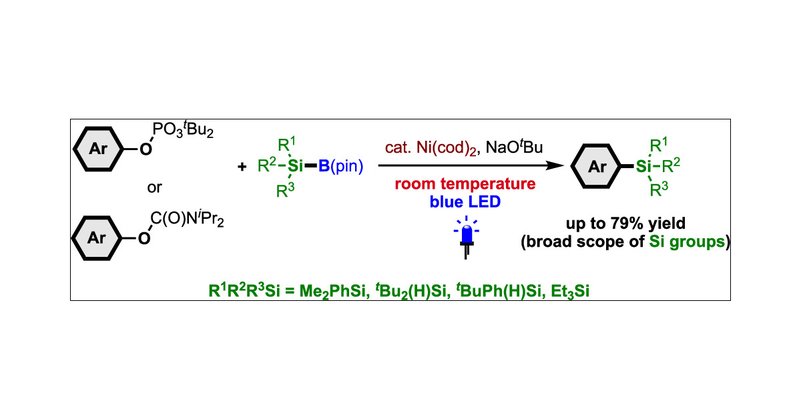
Our latest paper has been published in Org. Lett. @JOC_OL ! “Visible-Light-Driven Silylation of C(sp2)–O Bonds with Silylboranes via Nickel Catalysis” https://t.co/DiGferudIG
0
1
31
永島(A03)グループ学生の受賞 名前:飯室遥香(M2) 学会名:第51回反応と合成の進歩シンポジウム 賞:優秀発表賞(ポスター) 会期:2025年11月2日~3日 演題:長波長光を利用したラジカル型典型元素導入反応の開発
0
1
9
Our latest paper has been published in Org. Lett. @JOC_OL ! “Visible-Light-Driven Silylation of C(sp2)–O Bonds with Silylboranes via Nickel Catalysis” https://t.co/DiGferudIG
pubs.acs.org
Developing a general and efficient method for C(sp2)–O bond silylation that tolerates broad silyl groups under mild conditions remains a key challenge. Here, we report that naphthol and phenol...
1
4
54
製薬会社でメドケムをやっていた頃、担当した多くのプロジェクトでhERG阻害が課題になって苦労しました。分子の塩基性を下げれば解決しますが、大抵は標的活性も落ちてしまうというジレンマ…。
0
5
81
先日、第51回反応と合成の進歩シンポジウムにて「ホウ素錯体の光励起を利用する3次元化合物の合成法の開発」に関して、発表させていただきました。また、私のグループの学生さん(M2)がポスター発表賞を受賞しました! ディスカッションいただいた先生方、誠にありがとうございました😊
0
0
19
こちらの研究は、以前報告した研究(Nat. Commun. 2023 https://t.co/LswtapJBkD)を元に展開したものです。シリルボランにヘテロ環を加え光活性化できた経験から、次は触媒的に活用したいと考えました。フェノチアジンがそれに適切であることを見出し、本触媒系特有のケイ素化反応が実現できました。
nature.com
Nature Communications - Organoboron and organosilicon compounds have potential applications in various fields including materials science and medicinal chemistry. Here the authors report a protocol...
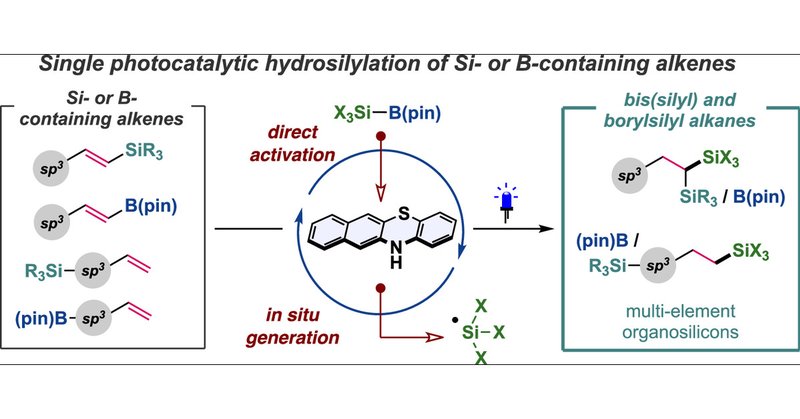
Our latest paper has been published in @JACS_Au ! Single Photocatalytic Hydrosilylation of Alkenes for the Synthesis of Bis(silyl) and Silaboryl Alkanes https://t.co/4j28P21oS8
0
1
23
Our recent paper has been selected as a cover @JACS_Au ! “Single Photocatalytic Hydrosilylation of Alkenes for the Synthesis of Bis(silyl) and Silaboryl Alkanes” JACS Au 2025, 5, 4481-4490. https://t.co/u1p9osxTn9
0
6
53
Our latest paper has been published in @JACS_Au ! Single Photocatalytic Hydrosilylation of Alkenes for the Synthesis of Bis(silyl) and Silaboryl Alkanes https://t.co/4j28P21oS8
pubs.acs.org
Bis(silyl) and silaboryl alkanes are of interest as bioactive compounds and highly functionalized synthetic building blocks, but conventional hydrosilylation reactions lack generality and/or select...
1
5
92
What was a turning point in Yuki Nagashima's career? Find out on his #IntroducingAngewandte page https://t.co/MKUhyVIz8q
https://t.co/mhbXHMLnyI
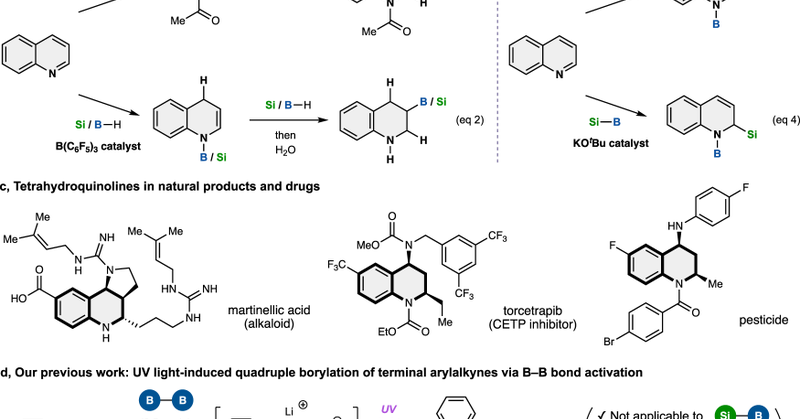
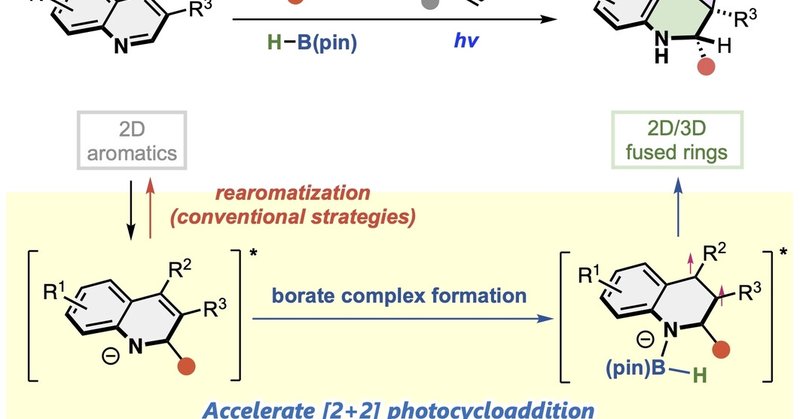
Dearomative Construction of 2D/3D Frameworks from Quinolines via Nucleophilic Addition/Borate-Mediated Photocycloaddition (Yuki Nagashima and co-workers) @KenTanaka_Lab
0
3
15
[Award Accounts] #ChemicalSpace , #ComputationalChemistry , element chemistry, organic #Photoreactions Article by Dr. Yuki Nagashima @UTokyo_News_en (The University of Tokyo) #Chemistry #OnTheCover #FreeAccess
https://t.co/mPsZctl2hg
1
3
13
#DFTcalculation , #GoldCatalysis , Intramolecular #Cyclization Article by Dr. Yuki Nagashima and Prof. Ken Tanaka @tokyotech_en (Tokyo Institute of Technology) #OrganicChemistry #Chemistry
https://t.co/fkmkKLcjIv
0
2
7
Dearomative Construction of 2D/3D Frameworks from Quinolines via Nucleophilic Addition/Borate-Mediated Photocycloaddition (Yuki Nagashima and co-workers) @KenTanaka_Lab
onlinelibrary.wiley.com
Dearomative construction of triply-fused 2D/3D frameworks was achieved from quinolines via nucleophilic addition and borate-mediated photocycloaddition in a chemo-, regio-, diastereo-, and enantios...
1
6
47
Now online: Article by Seiya Ouchi, Yuki Nagashima, Ken Tanaka and coworkers @KenTanaka_Lab Design, synthesis and visible-light-induced non-radical reactions of dual-functional Rh catalysts https://t.co/vpFTt0GS9t ($)
0
10
69
In this week's #NSynthPick we feature a new method for hydrostannylation and defluorostannylation using blue LEDs from Masanobu Uchiyama and Yuki Nagashima reported in @J_A_C_S
pubs.acs.org
We have developed photoboosted stannylation reactions of terminal alkynes (linear-selective hydrostannylation) and fluoroarenes (defluorostannylation), in which the stannyl anion is photoexcited to...
0
6
35