Vilde Kveim
@VildeKveim
Followers
84
Following
36
Media
7
Statuses
21
PhD student in the lab of @FlavioDonato82 @biozentrum
Joined August 2023
Excited to share that our paper is now published in @ScienceMagazine! You can check it out at https://t.co/BLAsxJPieT Thank you to everyone who made it happen! 🩷
science.org
Memories are dynamic constructs whose properties change with time and experience. The biological mechanisms underpinning these dynamics remain elusive, particularly concerning how shifts in the...
How can memories persist in the brain when their properties and supporting biological substrates change over time and experiences? Our latest work newly published in @ScienceMagazine proposes that a balance between memory dynamics and persistence is achieved through the
4
4
23
I feel incredibly honoured to be selected as an awardee of the J.C.W. Shepherd PhD Student Prize! ✨️ Thank you to the selection committee for this encouragement, and @FlavioDonato82 , colleagues, and the research community for their support! ❤️
Super proud PI moment today when my first PhD student @VildeKveim received the Shepherd price for her PhD work at today’s @biozentrum symposium @biozentrum_phds . So lucky to have started my lab with you, Vilde!!
0
0
1
The Donato Lab is at #SfN2024! Come by on Wednesday morning to see what we’ve been up to and discuss all things learning, memory, and development. @VildeKveim will present her latest results on how parallel encoding of multiple memory traces in developmentally defined neuronal
0
6
55
«During my master’s in neurobiology a lot of really exciting questions came up and as research is really fun for me, I decided to do a PhD and continue my research for a few more years”, explains Talia Ulmer. @biozentrum @UniBasel_en
https://t.co/ssADBRtJi6
0
5
29
BONUS: if you are at SfN, I will present these data on Sunday morning – poster number 109.01. Come and say hi! /end
0
0
1
This was of course a team effort and I’d like to thank @FlavioDonato82 @TaliaUlmer @spikesorting + (X-less) Laurenz Salm and Fabia Imhof for their support, insights, and enthusiasm! 13/
1
0
2
In conclusion, we discover that two distinct memory traces are established in the hippocampal network upon the encoding of a memory. They are instantiated in distinct populations of birthdated neurons, and recruited at distinct times to support memory progression over time. 12/
1
0
1
But that’s not all: manipulating EBNs or LBNs activity is sufficient to modulate the memory’s ability for such form of plasticity, thus revealing a potential memory-buffer trace and a suitable target to manipulate memory dynamics at will. 11/
1
0
1
First, we reveal that the recruitment of the transient LBN-dependent memory trace correlates with a short plasticity window when mice can integrate information across multiple learning sessions to reinforce learned associations or create inferences. 10/
1
0
0
But what are the functional implications of such divergent recruitment? Why create a transient trace? To answer this, we asked if the shift in recruitment from LBNs to EBNs could support changes in a memory’s propensity to be strengthened when new information is available. 9/
1
0
1
Do these two traces interact with each other? Yes! Preventing the establishment or consolidation of the transient LBN-dependent trace impairs EBN-dependent memory recall at remote times. 8/
1
0
1
Are these dynamics disruptive for memory processes? Not at all! In fact, the opposite is true: timely recruitment of EBNs/LBNs is necessary for the retrieval of the memory at specific delays from encoding. 7/
1
0
0
We thus hypothesize that multiple memory traces, each encoded in a distinct birthdated subpopulation, might be established at acquisition and alternatively recruited at recall, driving the progressive reorganisation of memory ensembles with time. What does this imply? 6/
1
0
1
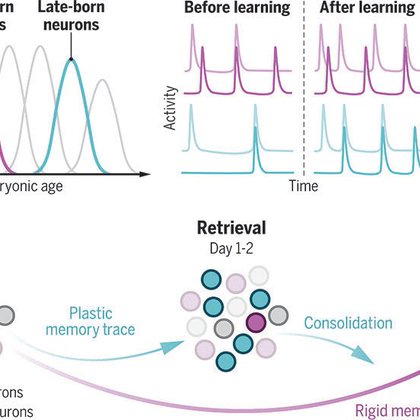
These dynamics align with those of early and late born engram neurons, and with longitudinal activity dynamics of the CA3 network. Calcium imaging in fact confirms that distinct neuronal ensembles are active for recall at 1d and 14d, if an associative memory has been formed. 5/
1
0
0
Though both populations are recruited similarly into memory ensembles at encoding, they exhibit distinct trajectories later on: upon recall, ensembles are enriched in LBNs in the first few days after acquisition, while EBN recruitment is delayed but sustained at remote times. 4/
1
0
0
To answer this, we investigated recruitment dynamics of neurons born early (EBNs) or late (LBNs) during neurogenesis to memory ensembles established during adulthood, and dissected their causal contribution to a memory’s evolution over time. 3/
1
0
0
We know that hippocampal neurons are functionally diverse, and that diversity is partially rooted in neurogenesis. Hence we wondered: can the dynamic recruitment of distinct subpopulations of birthdated neurons support dynamic changes a memory goes through in its lifetime? 2/
1
0
1
Memories, as well as their supporting neuronal ensembles, change with time and experience. But what is the logic of these processes and how do ensemble dynamics support changes in memory properties over time? 1/
1
0
1
Super excited to share my PhD work in @FlavioDonato82 lab! TL;DR: the dynamic recruitment of neurogenesis-defined hippocampal neurons into memory ensembles supports a memory’s persistence and modulates its plasticity over time. A 🧵
Divergent Recruitment of Developmentally-Defined Neuronal Ensembles Supports Memory Dynamics https://t.co/75w49sEFJY
#biorxiv_neursci
1
11
49
Hey, that's me! Come and discuss with us about those powerful little things that make us who we are - our memories! Sept. 26th and 7 pm. @biozentrum @UniBasel_en
First #EinblickeBiozentrumin in Autumn with @FlavioDonato82 How are memories formed and how do they change with time? Learn more about how the brain creates memories and how they shape the way we look at the world. @biozentrum Sept. 26 at 7 pm. https://t.co/zoTB40Kcdp
0
4
41