Eric Sidler
@SidlerEric
Followers
102
Following
152
Media
0
Statuses
38
SNF Postdoc Fellow in Ben Feringa's group. PhD with Marcel Mayor. Future PI and Ambizione Fellow at University of Bern.
Joined September 2020
A fully funded PhD position is available in my future research group in Bern @DCBPunibern (start between May and September 2026). We are developing polycyclic aromatics for semiconductors, sensors & more! Please spread the word! More information here:
eth-gethired.ch
1
5
12
A closer L👀K at the multiconfigurational ground state. BIG thanks to the whole co-author crowd for a wonderful collaboration!! @mishshan @IBMResearch @NanotechSurfLab @UZH_Chemistry @UZH_ch @JuricekLab @J_A_C_S
https://t.co/JRhtyqYd4E
2
25
128
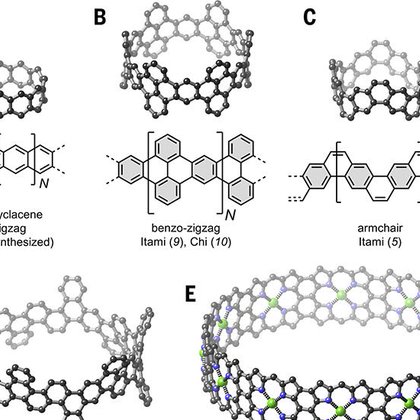
Latest from the @HLAGroupOx out now in @ScienceMagazine: Synthesis of triple stranded porphyrin nanobelts https://t.co/XL32oG6RYi Congratulations to all involved!
science.org
Molecular nanobelts are fascinating analogs of carbon nanotubes. Their rigid geometries and strongly coupled π-electrons have the potential to generate a wave function resembling that of a quantum...
2
67
239
👇 PhD position available 👇 Congratulations, Eric! Looking forward to your exciting research at the University of Bern! Happy we can have you with us before you launch your own group! @SidlerEric @unibern @UZH_Chemistry @snsf_ch #ambizione #newPI
Over the moon to be among the recipients of an Ambizione Grant!! Big thanks to @snf_ch, @unibern, @DCBPunibern and @albrecht_lab! We will synthesize polycyclic aromatics for semiconductors, sensors and optoelectronics!! Fully funded PhD position available! Repost appreciated!
0
2
10
Over the moon to be among the recipients of an Ambizione Grant!! Big thanks to @snf_ch, @unibern, @DCBPunibern and @albrecht_lab! We will synthesize polycyclic aromatics for semiconductors, sensors and optoelectronics!! Fully funded PhD position available! Repost appreciated!
3
7
22
Re-ver-si-ble CPL inversion at the molecular level promoted by light and heat! This collaborative work between the @FeringaLab and our group, the PulSAr team @UnivAngers, is now online on ChemArXiv. Special congrats to @HardoinLouis and Eric Sidler!! https://t.co/v098TQcnDa
0
12
90
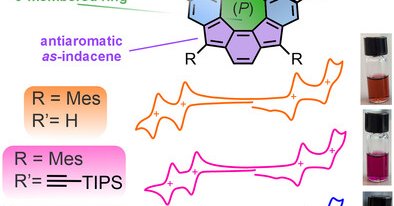
In our recent publication @angew_chem, we report the fusion of quasi[8]circulenes and indenofluorenes to get redox-active curved antiaromatics. Together with @RobertHeinChem and @FeringaLab! Check out my first publication as co-corresponding author here: https://t.co/BwMKrpzvnk
onlinelibrary.wiley.com
A new class of curved, chiral polycyclic aromatic hydrocarbon emerges from the fusion of indeno[2,1-c]fluorene and quasi[8]circulene motifs. These redox-active, antiaromatic scaffolds offer great...
1
3
49
Thank you Prof. Reisman for an inspiring course and your time to meet with students over lunch, dinner or coffee. A very special experience for us 😊🙏✨ @UZH_Chemistry @UZH_ch @Givaudan
👩🔬✨How often do you get the chance to interact directly with a world-leading chemist? Our students did just that with Professor Sarah Reisman from Caltech during the Givaudan–Karrer Lectureship! @UZH_Chemistry @Givaudan @NevadoLab @UZH_ch
0
2
11
Another great collaboration with @SidlerEric and @FeringaLab is now published in @angew_chem! Building on our previous work, Eric prepared a few large, formally antiaromatic systems with interesting (chir)optical and redox properties. Check it out here: https://t.co/qLVJBp3zmO
2
20
130
Check out our recent work in @angew_chem. It was an absolute pleasure to work on this phenomenal project together with @RobertHeinChem and Yohan Gisbert @FeringaLab. I am still absolutely amazed by the complexity and concurrent simplicity of this approach.
Chiral Induction and Memory via Supramolecular Deracemization (Ben L. Feringa and co-workers) @RobertHeinChem @SidlerEric @FeringaLab #openaccess 🔓
0
1
7
Chiral Induction and Memory via Supramolecular Deracemization (Ben L. Feringa and co-workers) @RobertHeinChem @SidlerEric @FeringaLab #openaccess 🔓
onlinelibrary.wiley.com
The helical chirality of a dynamic crown-ether switch can, by light- or redox-switching to a prochiral state, be erased and subsequently stereoselectively re-formed by relaxation in the presence of...
0
5
56
🔥 Hot of the press: Chiral induction and stable chiral memory via non-covalent host-guest binding now published in @angew_chem! One of my favourite projects and a super rewarding collaboration with @SidlerEric, Yohan and @FeringaLab! Hot paper here: https://t.co/gvif7qP1yM
onlinelibrary.wiley.com
The helical chirality of a dynamic crown-ether switch can, by light- or redox-switching to a prochiral state, be erased and subsequently stereoselectively re-formed by relaxation in the presence of...
3
16
77
Our collaborative research centre @uni_muenster on Intelligent Matter was funded for a further 4 years! Up to 15 PhD positions across various projects spanning chemistry, physics and related disciplines are available! Further details below/linked RT = ❤️ https://t.co/Zg6BnE9HPf
2
11
25
Wonderful idea and great progress on an unsolved long-standing challenge. Congratulations!
chemistry-europe.onlinelibrary.wiley.com
Herein we demonstrate the synthesis and characterization of a naphthalene-decorated [12]cycloparaphenylene acetylene. Subsequent benzannulation reactions on the acetylenes allow formation of an...
2
7
29
I am delighted to post about my main PhD project and our latest publication in @ChemEurJ! Here we demonstrate our efforts to synthesize a carbon nanotube via a bottom-up approach. Along the way, we also synthesized the longest cycloparaphenylene acetylene to date @UniBasel.
A Cycloparaphenylene Acetylene as Potential Precursor for an Armchair Carbon Nanotube ( @SidlerEric, Marcel Mayor and co-workers) #OpenAccess
3
6
21
A Cycloparaphenylene Acetylene as Potential Precursor for an Armchair Carbon Nanotube ( @SidlerEric, Marcel Mayor and co-workers) #OpenAccess
chemistry-europe.onlinelibrary.wiley.com
Herein we demonstrate the synthesis and characterization of a naphthalene-decorated [12]cycloparaphenylene acetylene. Subsequent benzannulation reactions on the acetylenes allow formation of an...
1
8
34
New Largest [n]Cycloparaphenylene Acetylene: Novel macrocycle shows bright turquoise fluorescence with a high quantum yield; might be a useful carbon nanotube precursor (work published in @ChemEurJ) https://t.co/Gn0BhUY036
0
10
37
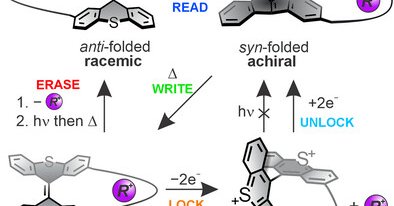
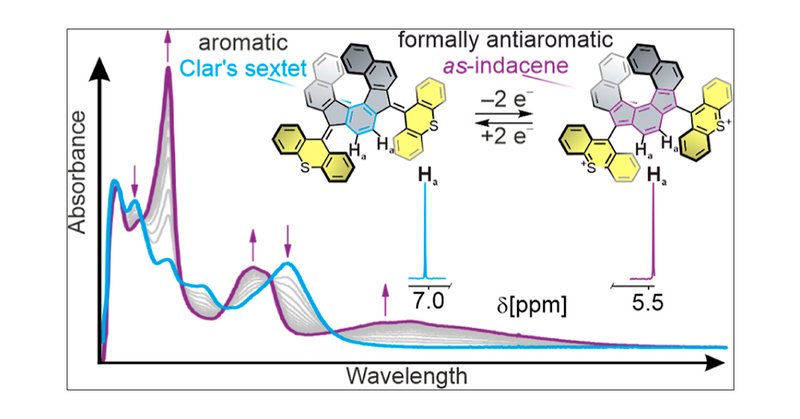
Happy to report my first Postdoc work of the @FeringaLab in @J_A_C_S, with @RobertHeinChem and @dan_doellerer. Here we demonstrate the highly reversible aromaticity switching in a chiral indenofluorene, owing to the dynamic redox system of bisthioxanthylidenes.
Redox-Switchable Aromaticity in a Helically Extended Indeno[2,1-c]fluorene | Journal of the American Chemical Society @StratinghInst @univgroningen #Redox #Switch #Aromaticity #Helical #Indenofluorene
4
7
33
Redox-Switchable Aromaticity in a Helically Extended Indeno[2,1-c]fluorene | Journal of the American Chemical Society @StratinghInst @univgroningen #Redox #Switch #Aromaticity #Helical #Indenofluorene
pubs.acs.org
Molecular switches have received major attention to enable the reversible modulation of various molecular properties and have been extensively used as trigger elements in diverse fields, including...
1
15
92
📢PhD Position available📢 I am looking for a PhD student to join my research group at the University of Münster! If you are interested in the development of novel redox switches then please get in touch or check out the job posting below. RTs appreciated! https://t.co/bniCSpdn9A
2
47
96