Nicholas Short MD
@NicholasShortMD
Followers
2K
Following
661
Media
8
Statuses
347
Associate Professor, Department of Leukemia, MD Anderson Cancer Center - Research interest: phase I/II clinical trials in AML and ALL, developing new MRD assays
Houston, TX
Joined February 2017
RT @DrHKantarjian: WBC >70K (but not IKZF1plus) increases risk of relapse with blina+ponatinib in Ph+ ALL, particularly extramedullary/CNS.….
0
30
0
RT @BloodAdvances: Measurable residual disease is a powerful predictor of clinical outcomes in acute lymphoblastic leukemia. .
ashpublications.org
Abstract. Measurable residual disease (MRD) is a powerful predictor of clinical outcomes in acute lymphoblastic leukemia (ALL). In addition to its clear pr
0
13
0
RT @DrHKantarjian: Expert recommendations on using MRD in #ALL now out in @BloodAdvances. ClonoSEQ (NGS MRD) is superior to other MRD metho….
0
39
0
RT @ALL_Hub_: Recommendations from a panel of US experts published in @BloodAdvances highlight the optimal use of MRD as a prognostic and t….
0
4
0
RT @DrHKantarjian: Early attainment of undetectable MRD by MFC is independently associated w/ improved RFS in newly diagnosed #AML. Interme….
0
37
0
Very nice of @AjHematology to add a wonderful visual abstract to accompany our review article on MRD in AML. Link here: @DillonHaem.
onlinelibrary.wiley.com
Answer questions and earn CME credit
MRD is highly prognostic in #AML and can inform SCT decisions or enrollment into MRD-directed clinical trials. MRD endpoints may also allow for accelerated drug approval in AML. New review by @NicholasShortMD and Richard Dillon in @AjHematology .#leusm
3
16
82
RT @ALL_Hub_: CONGRESS #ASH24 | PRESENTATION.@WNMacaron, @MDAndersonNews and @bcmhouston discussed achievement of MRD negativity and posit….
0
7
0
RT @ALL_Hub_: CONGRESS #ASH24 | PRESENTATION.Roberta Santos Azevedo, @MDAndersonNews discussed older age and TP53 mutations as predictors….
0
5
0
RT @AML_Hub: CONGRESS | #ASH24 | PRESENTATION.@NicholasShortMD @MDAndersonNews shares long-term findings from a study of patients with ND F….
0
10
0
RT @hemoncer: Save the Date! @calliecoombsmd @JohnPLeonardMD @Ramikomrokji @farrukhawan @NicholasShortMD @szusmani @DrKrinaPatel @corinneSh….
0
6
0
RT @DrHKantarjian: MRD is highly prognostic in #AML and can inform SCT decisions or enrollment into MRD-directed clinical trials. MRD endpo….
0
42
0
Happy to see our trial of DAC, venetoclax and ponatinib for advanced phase CML published in @TheLancetHaem. Response rate was 80%, allowing for bridge to alloSCT. Still a lot more work to be done in this rare disease. We have new TKI combo studies now open and enrolling.
In a Phase II trial led by Dr. Nicholas Short, 80% of patients with previously untreated chronic myeloid leukemia or Philadelphia chromosome-positive acute myeloid leukemia achieved remission with a novel treatment combination: @NicholasShortMD #EndCancer.
4
11
70
RT @DrHKantarjian: NGS MRD (clonoSEQ) is the most sensitive and specific MRD assay in Ph+ #ALL. Therapeutic decision-making should be based….
tandfonline.com
Published in Expert Review of Hematology (Vol. 17, No. 6, 2024)
0
24
0
Our new review on inotuzumab ozogamicin is now online. We cover the clinical development of INO, pivotal studies, and the novel uses of INO in B-ALL, including combination therapies and use in the frontline setting.
Inotuzumab approved as monotherapy for R/R #ALL but new research is expanding its use into frontline, for MRD+, and in combo with blinatumomab. Best outcomes seen in combinations. @NicholasShortMD of @MDAndersonNews #Leukemia.#leusm
4
1
29
RT @Satyayadav__: Listening to #NicholasShort from @MDAndersonNews talking about #inotuzumab in relapsed refractory Acute lymphoblastic….
0
3
0
RT @DrHKantarjian: Many pts with Ph+ #ALL can be salvaged with INO, blina or CAR T-cells after ponatinib failure. Long-term remissions poss….
pubmed.ncbi.nlm.nih.gov
Characteristics and outcomes of patients with relapsed Philadelphia chromosome-positive acute lymphoblastic leukemia after failure of a frontline ponatinib-containing therapy
0
15
0
RT @Anand_88_Patel: A very nice paper in @BloodJournal elucidating the mechanisms of resistance to inotuzumab in B-ALL that go beyond loss….
0
18
0
RT @FDAOncology: FDA granted accelerated approval to ponatinib with chemotherapy for adult patients with newly diagnosed Philadelphia chrom….
fda.gov
On March 19, 2024, the Food and Drug Administration granted accelerated approval to ponatinib (Iclusig, Takeda Pharmaceuticals U.S.A., Inc.) with chemotherapy f
0
29
0
RT @doctorveera: Someone used DALL-E to create gobbledygook scientific figures and submitted them to Frontiers Journal. And guess what? The….
0
2K
0
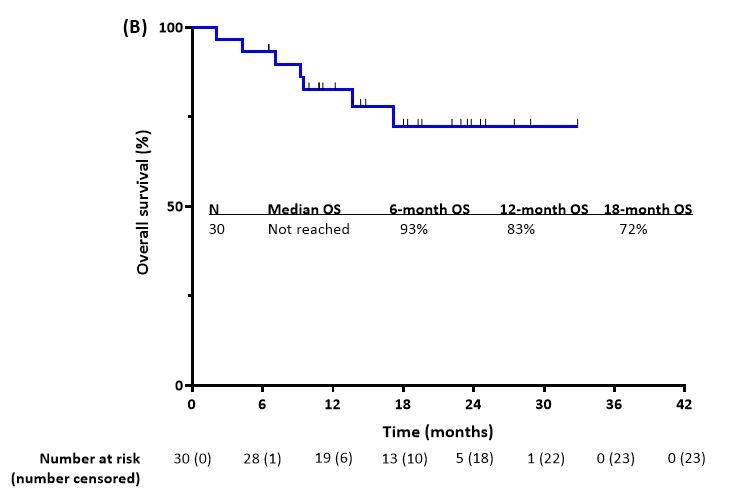
Our paper on aza + ven +gilteritinib for FLT3mut #AML now online at @JCO_ASCO.In frontline FLT3mut AML, CR 90% (vs. 30-50% with aza+ven) and 18mo OS 72% (vs. 2yr OS 20-40% with aza+ven).Could be delivered safely with appropriate dose modifications.
ascopubs.org
PURPOSEAzacitidine plus venetoclax is a standard of care for patients with newly diagnosed AML who are unfit for intensive chemotherapy. However, FLT3 mutations are a common mechanism of resistance...
Aza + ven + gilteritinib highly effective in newly dx older FLT3-mut #AML: CR/CRi 96% and 18-month OS 72%. A new standard of care in this population. @NicholasShortMD of @MDAndersonNews #Leukemia.@JCO_ASCO .#leusm
5
23
96