David S. Hong MD
@DavidHongMD
Followers
4K
Following
6K
Media
324
Statuses
2K
MD Anderson Cancer Center medical oncologist, Deputy Chair of Dept. Investigational Cancer Therapeutics. Phase I clinical trials.Tweets are mine.
Houston, TX
Joined February 2016
Crossing the Bridge!.
It’s awesome being here in Houston. Finished up a clinical advisory board meeting for @CrossBridgeBio that included @DavidHongMD @DoctorDietrich Funda Meric-Bernstam et al! Excited for CBB120 and the opportunity to truly unlock the Trop2 opportunity. Bonus was time with Tim Yap!.
0
1
5
Roger Roger!.
Fantastic lecture by @DavidHongMD on targeting KRAS in #PancreaticCancer. @MDAndersonNews has the largest portfolio of KRAS inhibitor trials (👀 slide 1!), his favorite G12C “children”, how KRAS inhibitors are stacking up against chemotherapy, and of course Roger Bannister!
0
1
11
We will miss you and you will miss the BBQ! @MDAndersonNews 🥹.
One last goodbye on Sunday night to the place that was home for the last decade. The #PancreaticCancer research center was the very first occupant of this brand new research building. Time flies!
0
0
12
Checkout Telperian! It really is the wave of how clinical trials will be designed and data will be analyzed!
linkedin.com
🚀 We’ve just launched our new website! 🚀 We’re thrilled to unveil the brand new telperian.com — redesigned from the ground up to better reflect who we are and how we help our partners transform...
0
1
5
Another ring!.
🎉🎉🎉We celebrate #FDA approval of #zongertinib as the first TKI in #HER2-mutant #NSCLC 🎊 🎂 🌺 🩷 .👉 ORR 71% .👉 Duration of Response 14.1 months .👉 PFS 12.4 months .👉 Well-tolerated at 120mg dose.📖 In previously treated patients without prior anti-HER2 targeted therapies
0
0
14
This combo needs to be done!!.
Targeting the YAP-SDC1 axis overcomes resistance to KRASi in GI cancers by blocking SDC1-driven macropinocytosis and RTK activation, offering a promising strategy for KRASi resistance. @wantong_yao @HaoqiangY @CellRepMed #crcsm To the clinic!
1
1
17
Congrats Dr Torrado! And welcome to the @Mdanderson faculty!.
Marking my last day in clinic as a fellow. Incredibly grateful for two meaningful years in the Investigational Cancer Therapeutics Department at MD Anderson—especially to the patients, mentors, and colleagues who made the journey unforgettable.
1
2
14
RT @rkalluriMDPhD: Session 3.🔹Bob Wolff on biology to clinical implementation.🔹@Val_LeBleu on iExosomes therapy.🔹@DavidHongMD on the RAS Re….
0
1
0
Welcome Dr Gouda we are proud of you! @MDAndersonNews.
Effective today, I am starting a new role as an Assistant Professor of Medical Oncology at MD Anderson Cancer Center. My primary clinical focus will center on treating patients with melanoma; while continuing research aimed at advancing liquid biopsy and drug development in the
1
0
14
RT @mgoudaMD: Effective today, I am starting a new role as an Assistant Professor of Medical Oncology at MD Anderson Cancer Center. My prim….
0
12
0
RT @skopetz: Precemtabart tocentecan, an anti-CEACAM5 ADC, is active in heavily pretreated metastatic colorectal cancer—median PFS is 6.9 m….
0
15
0
Amazing science, Amazing colleagues, and Amazing beer! At the Mildred Scheel Cancer conference in Bonn Germany! @MDAndersonNews @KRASKickers
0
0
13
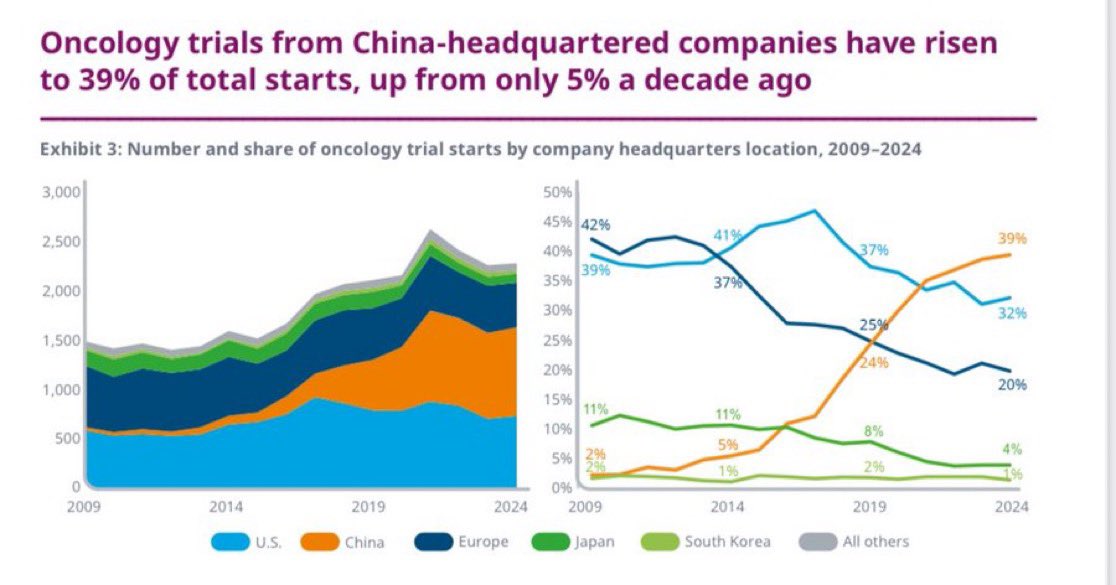
I need to learn mandrin! 😜.
Had a conversation with someone outside of healthcare recently who was shocked by how long it takes to run clinical trials in the U.S (not to mention expensive). I tried to explain—it’s not just the science. It’s the infrastructure. Every site needs dozens of personnel, many of
2
1
9
RT @GIMedOnc: Had a conversation with someone outside of healthcare recently who was shocked by how long it takes to run clinical trials in….
0
47
0
Yep! I say we just have the drug development session at ASCO in Mandarin 😆.
China has now surpassed all other countries in number of new oncology clinical trials launched each year, AND also number of novel active substances (NAS) for cancers launched. @IQVIA_global .
1
2
19