IMI_SISAQOL
@sisaqol
Followers
250
Following
39
Media
52
Statuses
134
Setting International Standards in Analysing Patient Reported Outcomes and Quality of Life Endpoints in Cancer Clinical Trials
Joined February 2021
@ISPORorg @sillytenseldam 📍 Poster 2: PCR135 Improving Patient Involvement in Cancer Clinical Trials: Development of Plain Language Checklists Within the SISAQOL-IMI Initiative. 🔸️ @sillytense from @MyelomaEurope on behalf of @WECANadvocate
0
0
0
SISAQOL-IMI is at @ISPORorg Europe 2025 presenting two posters in Glasgow, Scotland 🏴 #isporeurope #isporeurope2025 #sisaqol #qualityoflife #patientreportedoutcomes
2
0
0
We’re thrilled to announce that our consortium will be presenting the SISAQOL-IMI recommendations at the @RoyalStatSoc Conference 2025 in Edinburgh this September! We can't wait to share our insights and engage in thought-provoking discussions. Stay tuned for more details 📊📢
0
0
0
🚨 New Publication Alert! 🚨 Our latest publication in BMC Medical Research Methodology explores handling missing patient-reported outcome data in the presence of intercurrent events. Aligning analysis with the estimand framework is key! 📊 Read more:
bmcmedresmethodol.biomedcentral.com
Introduction As patient-reported outcomes (PROs) are increasingly used in the evaluation of medical treatments, it is important that PROs are carefully analyzed and interpreted. This may be challen...
0
0
0
Early this month, we presented key highlights from the final SISAQOL-IMI recommendations. We invite you to explore the executive summary about the public launch and the collaborative efforts that underpin this initiative https://t.co/EwWVTBlcMl
0
0
2
📢 Yesterday, SISAQOL-IMI officially launched its final recommendations for standardising patient-reported outcomes in cancer clinical trials! 🎉 A huge step toward better, more reliable data for patient-centered care. Learn more:
sisaqol-imi.org
SISAQOL-IMI Launches Final Recommendations to Enhance Patient-Reported Outcomes in Cancer Clinical Trials Ghent, 5 February 2025 – SISAQOL-IMI (Setting International Standards of Patient-Reported...
0
3
5
📢Today at the #SISAQOLIMI launch, MPE´s Silene ten Seldam will present on patient involvement and Plain Language Checklists for myeloma trial protocols. MPE board member, Vincent Claus also attended and joined a panel on implementing the recommendations.
0
1
2
Stakeholder panel at #SISAQOL. Interesting discussion about whether collection of PROs post-progression is feasible, how to make PROs less burdensome, & where to place PROs in the hierarchy of clinical trial endpoints. Universal agreement that SISAQOL moves the field forward!
0
2
2
Coming soon from #SISAQOL: guidebook, interactive table, glossary, patient material, and more! Will be posted on the SISAQOL website. https://t.co/Pck88hR4lu
0
1
2
Coming soon from #SISAQOL for patient advocates & patient research partners: checklist for reviewing PRO measures in protocols, and checklist for reviewing visualizations in cancer clinical trials.
0
3
5
#SISAQOL developed recommendations on how to select a threshold for interpreting PROs and how to report that threshold.
0
1
3
Harmonized terms to replace the vague term “MID” from #SISAQOL! Need to be clear about whether you are talking about within-patient change (eg, responder analysis) or three group-level options (mean comparisons within or between groups - see slides).
0
1
4
Excited that #SISAQOL included recommendations about #dataviz for #patientreportedoutcomes! Graphs vary by audience consistent with prior literature. Include p-values for preplanned tests only, and use consistent y-axis scaling throughout your paper.
0
3
7
Next #SISAQOL turns to #patientreportedoutcomes in single-arm trials. PRO objectives should be clearly defined & can be descriptive (more common) or comparative to historical control data. Important to consider missing data & incurrent events as in randomized trials.
0
2
4
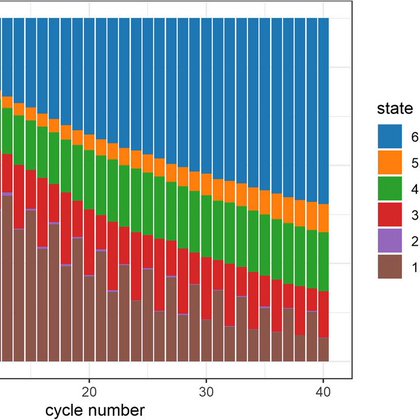
Next #SISAQOL highlights involve missing data. Need to separate missing data from intercurrent events, and recommendations reinforce using “completion rate” & “available data rate” - quit using “compliance rate” in your papers! Finally, avoid biased missing data approaches.
0
1
3
Diving into #SISAQOL recommendations. First highlights involve handling death or other intercurrent events. Basically there is no standard approach but need to be clear in the approach that you are using. Some new language here that many folks might not be familiar with…
0
1
3