Rita Kessler
@rkessler05
Followers
262
Following
198
Media
11
Statuses
870
European Advocacy Officer @RevuePrescrire Working on EU health & pharma policy. Views are my own.
Joined February 2018
RT @DTB_BMJ: Help save GeBu, the Dutch independent bulletin on medicines!. Geneesmiddelenbulletin (GeBu) provides independent and objective….
0
1
0
RT @consiliumsci: Join Consilium for another discussion panel on Thursday, 3rd October!. ❓ Whistleblowing and Whistleblowers in Medical Res….
0
3
0
RT @EUCourtPress: #EUGeneralCourt: The @EU_Commission did not give the public sufficiently wide access to the purchase agreements for #Covi….
0
139
0
RT @rozscourse: 🚨🚨NEW: MSF Access Campaign @MSFAccess report: Secrets Cost Lives – Transparency and Access to Medical Products. This report….
0
60
0
International Conference on Deprescribing (September 26-27, 2024, Nantes, France).With interventions by ISDB members (International Society of Drug Bulletins):.➡️WS 7: CBiP-BCFi, Belgium.➡️WS 12: Therapeutics Initiative, Canada.
📣 ICOD2: Registrations now open!📣. In-person or virtual attendance, early-bird registration fee until June 15th. More detailed info on the scientific program to come soon…. 👇👇. ⚠️ limited places ⚠️.
0
1
1
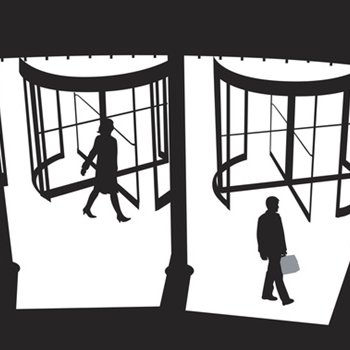
Revolving door: You are free to influence us “behind the scenes,” FDA tells staff leaving for industry jobs
bmj.com
Internal emails show that the US Food and Drug Administration informs employees leaving for industry jobs that, despite restrictions on post-employment lobbying, they are still permitted to influence...
0
1
1
France: La HAS souhaite une réflexion sur la doctrine d’évaluation d'autorisations d’accès précoce pour apprécier l’incertitude, sur le bénéfice clinique du médicament, entourant la demande.
has-sante.fr
Autorité publique indépendante à caractère scientifique, la Haute Autorité de santé (HAS) vise à développer la qualité dans le champ sanitaire, social et médico-social, au bénéfice des personnes....
0
0
2
RT @gerdantes: Unveröffentlichte Studienergebnisse gefährden die evidenzbasierte Gesundheitsversorgung!.
0
26
0
RT @beuc: The EU’s pharmaceutical legislation is undergoing its biggest reform in two decades. Now is the moment for member states to pay….
blog.beuc.eu
The EU pharma reform is the moment for member states to pay particular attention to what consumers need, writes Ancel.la Santos.
0
1
0
14 associations françaises publient leur ordonnance pour une nouvelle politique du médicament. Avec une contribution de @RevuePrescrire sur la révision de la législation pharmaceutique européenne.
@Fr_Assos_Sante et 13 associations publient leur ordonnance pour une nouvelle politique du médicament. 𝐋'𝐨𝐛𝐣𝐞𝐜𝐭𝐢𝐟 :.🚨 alerter sur les difficultés d’accès.💊 proposer des solutions concrètes.💪 inciter à la mobilisation.
0
0
0
Have a look at @EMA_News public consultation on guidance document on the identification of personal data and commercially confidential information within the structure of MA application dossier (deadline 28 June)
0
0
1
RT @arzneitelegramm: Geheime #Erstattungsbeträge im #Gesetzentwurf des #Medizinforschungsgesetzes (MFG) - mit MFG assoziieren wir „Mit freu….
arznei-telegramm.de
Mit dem Ende März 2024 vom Bundeskabinett beschlossenen Gesetzentwurf des Medizinforschungsgesetzes (MFG) kommt das Bundesgesundheitsministerium (BMG) langjährigen Forderungen der Pharmaindustrie
0
1
0
RT @consiliumsci: If you missed a talk at Consilium by @TheWonkologist last Thursday, watch it here: @drkevinknopf….
0
1
0
Prescrire's ratings of new drugs in 2023.@PrescrireInt , @RevuePrescrire. ▶️Bravo : 0 % .▶️A real advance: 0 % .▶️Offers an advantage: 8 %.▶️Possibly helpful: 16 % .▶️Nothing new: 60 % .▶️Judgement reserved: 8 %.▶️Not acceptable: 7 %.
english.prescrire.org
Spotlight
0
10
12
RT @DTB_BMJ: Do medicine safety warnings change clinical practice?.In her latest editorial, Barbara Mintzes discusses the impact of regulat….
0
1
0