Ravi Gupta
@rgupta729
Followers
484
Following
4K
Media
23
Statuses
1K
doctor and health policy @HopkinsMedicine
Joined November 2008
ICMYI: AI/ML-enabled medical devices have potential for earlier diagnosis & symptom monitoring for Alzheimer’s disease & other dementias. A new @JAMA_current study led by @HopkinsMedicine student Krista Chen looks at demographic representativeness of these devices (1/4)
The @US_FDA's authorization of #AI devices for #Alzheimer disease is promising but lacks transparency in demographic data, risking algorithmic bias and inequitable application across diverse patient groups. https://t.co/ekbmIy4ehv
#AAIC2025
1
1
2
In their new Forefront article, @EstherOh_MDPhD, @Rock1MD, @RealJoeGrogan, @rgupta729, Anne Ollen, @healthecon_dan, Peter Weems, and Phillip Phan from @HopkinsMedicine and @USCSchaeffer discuss how creating clear regulatory frameworks and reimbursement standards can help ensure
healthaffairs.org
Creating clear regulatory frameworks and reimbursement standards can help ensure the responsible and effective integration of AI to improve the health of older adults.
1
2
1
Looking forward to talking about prior authorization with A. Mark Fendrick, Ravi Gupta, and Michael Anne Kyle at Health Affairs’ upcoming Insider Event, "Prior Authorization: Current State and Potential Reform," on September 23 from 1 pm – 2 pm ET. Sign up using the link below.
1
2
6
@JHU_HBHI The 09/17 HEADS Center Seminar will feature @JHUNursing assistant professor @AbshireMartha, @BSPH_HPM assistant professor @rgupta729, and @JHU_HBHI affiliate trainee Zhang Zhang.
0
1
2
New @HopkinsMedicine @Hopkins_GIM research in @JAMACurrent ( https://t.co/rnetyhdxGC) urges better transparency of demographic composition of data used to develop FDA-authorized #AI-based devices for diagnosis/management of #AlzheimersDisease & related #dementias. @rgupta729
jamanetwork.com
This cohort study examines the availability and representativeness of data supporting US Food and Drug Administration–authorized artificial intelligence and machine learning devices to treat Alzhei...
0
3
6
High brand-name drug prices fall once a generic enters the market. In a new @JournalGIM article, @rgupta729, CRRIT Co-Director @jsross119, and colleagues from @PORTAL_Research assess associations between patents, revenue, and generic competition.
1
4
7
0
0
2
More work is needed on the effect of PACE program ownership status on patient outcomes, particularly given growing evidence on the consequences of private equity for patient health.
1
0
3
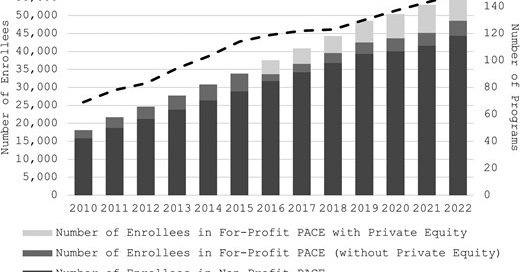
We found that after the rule change, while overall program growth and enrollment actually slowed, the number of PACE enrollees receiving care from for-profit programs, often with private equity investment, increased primarily due to the entry of new programs.
1
0
0
Historically, PACE programs have been operated by non-profit entities, but a 2016 rule allowed for-profit PACE programs, which may grow PACE by enticing investments and private equity, but with uncertain effects on patient health and outcomes.
1
0
0
PACE is a managed care program financed by capitated government payments that serves adults >55 years who are dual eligible for Medicare and Medicaid and require nursing home level of care. PACE aims to help people meet their needs in the community instead of a nursing home.
1
0
0
We have new work led by Katherine Miller and mentor @healthecon_dan in @Health_Affairs Scholar on the role of for-profit programs and private equity in PACE (Program of All-Inclusive Care for the Elderly): https://t.co/1ItJXaGpex
academic.oup.com
Abstract. The Program of All-Inclusive Care for the Elderly (PACE) is a managed care program financed by capitated government payments that primarily serve
2
3
14
🚨EDs are critical for persons living with dementia (PLWD). Could EDs be integrated into the CMS GUIDE Model to improve dementia care? Learn more in a new @Health_Affairs article by @HashemZikry @SMDresdenMD @rgupta729 and Ari Friedman : https://t.co/n43l0TwnyH 🧵 (1/8)
healthaffairs.org
Consistent with the approach taken by other alternative payment models, GUIDE frames the emergency department as a negative outcome to be avoided. In doing so, GUIDE ignores the reality on the ground...
1
2
4
The opioid industry's use of scientific evidence to advance claims about prescription opioid safety and effectiveness https://t.co/R7RQC67B8H via @rgupta729 et al
academic.oup.com
Abstract. It is widely recognized that pharmaceutical marketing contributed to the ongoing US opioid epidemic, but less is understood about how the opioid
0
2
6
Excited to share my new paper Health Affairs Scholar with Mingqian Liu, @jeromie_b , @rgupta729 and Jerry Anderson on Biopharmaceutical pipeline funded by venture capital firms, 2014 to 2024
academic.oup.com
Abstract. Venture capital (VC) firms fund biopharmaceutical research and development (R&D) while incurring substantial financial risk. VC firms seek to
1
4
9
This work was a joint effort with Jason Chernesky, Caleb Alexander, Anna Lembke, @AdamKoon @DrTomori @drdavidmichaels @jgreene2 Summary here as well by @JohnsHopkinsSPH:
lnkd.in
This link will take you to a page that’s not on LinkedIn
3
2
3
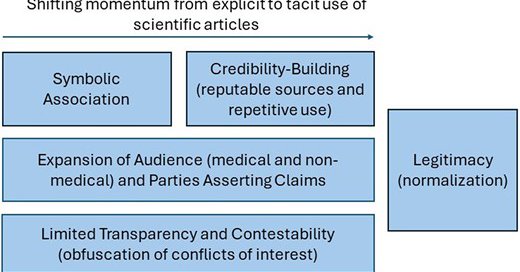
We used documents from the UCSF-JHU Opioid Industry Documents Archive @industrydocs to characterize specific industry strategies that allowed unsubstantiated claims to gain legitimacy by the misrepresentation of a scientific foundation to disarm criticism & promote opioid sales.
1
0
3
*New work* in Health Affairs Scholar: https://t.co/7ZZiUGJ3E8 We investigate how the opioid industry used scientific articles to influence prescribing of prescription opioids and shape unsubstantiated claims about the benefits of opioids and manufactured doubt about their risks.
lnkd.in
This link will take you to a page that’s not on LinkedIn
3
4
7
Some of my favorite lawyers on a panel for the @drsforamerica FDA Pre-Conference at #NLC2024 - Matt Tracy, former @ColumbiaLaw student of @cmorten2, Director of the Science, Health, & Information Clinic and Ben Seel of @DemocracyFwd on ongoing court challenges to FDA authority.
3
9
17