Michael Reese Lab @ UTSW
@reeselab1
Followers
620
Following
722
Media
32
Statuses
707
The Reese Lab at UT Southwestern Medical Center studies the human parasite #Toxoplasma. Opinions are my own (or do they belong to the parasites in my brain...?)
Dallas, TX
Joined February 2019
Moving to Mastodon. Much better fit. Should anyone want to find me I'm @notoriousiptg@fediscience.org
0
1
6
The lab that wishes we were a band
0
0
28
Thanks! It's an honor to join such a distinguished group of scientists!
Congratulations to @reeselab1!
10
1
33
What's cool to me is we can SEE these things in cells that are essentially captured in suspended animation. We're doing structural biology in a way I never thought possible when I was an undergrad/grad biophysics student! It's a fun time to be doing science.
0
0
4
We see that the microneme is docked just beside its partner organelle, the rhoptry, and both appear anchored to the same structural "ridge". Of course, we have no idea (yet) what the proteins involved are, but I'm excited for the community to find out!
1
0
2
There's a lot more cool things we discuss in the paper, but I'll just leave you with one more -- we captured a parasite "microneme" in the act of docking with the plasma membrane and secreting!
1
0
5
We also identified filaments that look a lot like actin that connect the conoid and the APR in the "out" conoids, suggesting that actin may be helping power these movements (magenta in the cytoskeleton overview image above).
1
0
3
Instead, we noticed the ring through which the conoid moves, called the apical polar ring (APR), appears to be flexible and dilate like an iris, expanding ~15-25% during movements. This appears to be because its coupled to the parasite membranes, which slide "up" with the conoid.
1
0
3
Some data is out there that shows differences in the conoid shape in "in" versus "out" states. But those studies used thin ice and pancaked samples. When we checked with our cryoFIB-milled samples that are as unperturbed as you can get, we saw no change in the conoid at all!
1
0
2
One open quesiton is what provides the force for the apical complex movements. The core of the Toxo apical complex is a spiral of tubulin called the "conoid." It looks like a spring, so people have suggested it might act like a spring, and compress and store/release energy...
1
0
2
That meant instead of messed up squished invasion machinery (or cells),we get nice happy cells in a native state. Awesome! This allowed us to capture the invasion machinery as it moved, and look closely at the parts that make it up, membranes and all!
1
1
3
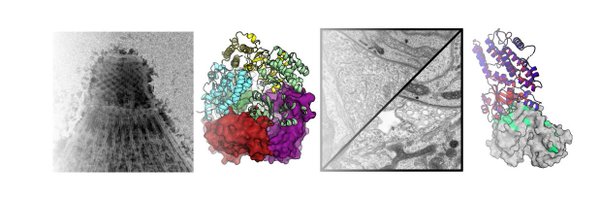
To get around this, we used cryoFIB milling, which uses a beam of ions to mill away all but a thin layer of your sample (called a "lamella")). It looks like this for our parasites...
1
0
2
One difficulty with cryoET is that you need samples to be super thin (like 100-200 nm), and if you use really thin ice, the samples squish into pancakes, which destroys the structure. The strength of the surface tension of water can even squish cells, cytoskeleton and all!
1
0
2
Apicomplexan parasites, cause some of the world's most devastating diseases, like malaria. One thing that makes these parasites so successful is their ability to move around and quickly invade their host cells. To do this they use a conserved machinery called the apical complex
1
0
2
After a few years of hard work, our cryoFIB/#cryoET of the #apicomplexan #parasite invasion machinery is finally on bioRxiv! This is in close collaboration with the Nicastro lab here at UTSW (not on twitter).
biorxiv.org
The apical complex is a conserved cytoskeletal structure that organizes the secretory and invasion machinery of all apicomplexan parasites, the causative agents of diseases such as malaria, toxopla...
8
14
95
Good week so far, our paper with @ToxInLa and @peter_s_back is out in @mbiojournal and I just heard I've been recommended for tenure! Time to take the lab out for lunch...
36
3
108
This was a really satisfying project, both because we were able to iron out a mechanism for a perplexing phenotype and because the answer was a huge surprise. Science is most fun when you are kept on your toes! Great work by a team led by Wil O'Shaughnessy & @estherhu1122
0
0
3
Normally, CSAR1 recycles the mother cytoskeleton after division, which is never turned over in Δcsar1. You can see this in the movie. This leads to lots of screwy things in these parasites, including daughter cell counting during division (notice 3 and 4 buds in movie).
1
0
0
We were able to follow this in live cells with GFP-tubulin. The bright dots are the "conoid" of the apical complex that disappear from the moms and babies ERK7 is lost (+IAA), but not when CSAR1 has been deleted!
1
0
0
Remarkably, deleting CSAR1 completely suppressed the ERK7 loss-of-function phenotype. The baby Toxo formed normal apical complexes and were able to invade new cells just fine. So ERK7 -| (inhibits?) CSAR1 -> apical complex not destroyed!
1
0
0