Paul Kluetz
@pkluetz
Followers
1K
Following
1K
Media
7
Statuses
897
med onc, interested in trial design, endpoints and scientifically rigorous measurement of the patient experience- tweets and opinions are my own
Joined December 2008
RT @FDAOncology: FDA today issued draft guidance, Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Popula….
0
3
0
RT @FDAOncology: TODAY: Join for the FDA 9th Annual Clinical Outcome Assessment in Cancer Clinical Trials (COA-CCT) Workshop with opening r….
0
2
0
RT @tmprowell: @VivekSubbiah @pkluetz @US_FDA @FDAOncology @montypal @weldeiry @Transplant_Doc @doctorpemm @BldCancerDoc @ErikaHamilton9 @c….
nejm.org
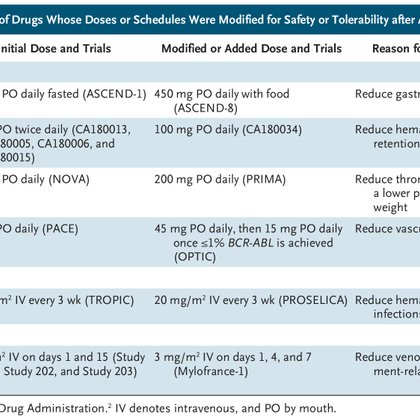
Doses and schedules of oncology drugs are sometimes inadequately characterized before registration trials. The “more is better” paradigm is still used for dose selection, despite the recognition th...
0
2
0
RT @FDAOncology: Congratulations to Kelly Norsworthy, MD, @KellyNorsworth2 on her new position as Division of Hematologic Malignancies 1 de….
0
2
0
Please join if you can. Patient generated data to inform the tolerability of drug products… exploring single item overall side effect impact with some fantastic experts in the #PRO field.
TODAY: Join for the FDA 8th Annual Clinical Outcome Assessment in Cancer Clinical Trials (COA-CCT) Workshop with opening remarks by OCE Deputy Director @pkluetz.#OCEOutcomes23.Event details:
0
2
12
This session did a nice job hearing both sides of the story on early endpoints and accelerated approval in oncology. Kudos to @FDAOncology Angelo De Claro for his balanced perspective.
1
1
16
Open access article in @TheCancerLetter worth reading. Discusses @FDAOncology Rick Pazdur's perspective on the oncology #drugshortage.
Read an interview with OCE Director Richard Pazdur about causes of oncology drug shortages and what the FDA can and can't do to fix it, via @TheCancerLetter open access article.
0
3
4
RT @FDAOncology: #ONSCongress 4/26- 3:15-5:15p CT: Hemisfair Ballroom 1. Meet @FDAOncology nurses!.FDA 101 and Nursing Roles in Unexpected….
0
1
0
The ORISE fellowship program has been such a productive opportunity for early career scientists to work with FDA on applied research questions @FDAOncology @Meena_PharmD.
Measuring Frailty Using Patient-Reported Outcomes (PRO) Data: A Feasibility Study in Patients with Multiple Myeloma. First author @Meena_PharmD, last author @pkluetz .#OCEPublications.
0
0
5
Excited to kick off this year's great line up of @FDAOncology regulatory science sessions by moderating an #AACR23 panel on #DecentralizedTrials for #Oncology #ClinicalResearch. Looking forward to seeing everyone in Orlando! Session details here
Adaptations to the #COVID19 pandemic showed that decentralized oncology trials are feasible. On Sunday, April 16, @FDAOncology Deputy Director Paul Kluetz will moderate a panel discussion on how to make these changes permanent at #AACR23.
0
1
18
RT @FDAOncology: Applications due April 3 for #FDA #RFA for Applied Regulatory Science Research to Understand Factors that Affect the Safet….
0
2
0
RT @FDAOncology: FDA Issues Draft Guidance Aimed at Improving Oncology Clinical Trials for Accelerated Approval . #….
0
12
0
RT @FDAOncology: Irreconcilable Differences: The Divorce Between Response Rates, Progression-Free Survival, and Overall Survival. Commentar….
ascopubs.org
0
16
0
RT @pmishrakalyani: Incredibly excited to share a very informative #FDA guidance on #externalcontrols hot off the presses! So proud to hav….
fda.gov
Considerations for the Design and Conduct of Externally Controlled Trials for Drug and Biological Products
0
14
0
RT @FDAOncology: We enjoyed meeting with Heme/Onc Fellowship Program Directors from @NorthwesternMed @Yale @BrownUniversity @universityofky….
0
3
0
RT @FDAOncology: Use of Single-Arm Trials for US Food and Drug Administration Drug Approval in Oncology via @JAMAOn….
0
6
0
RT @BiostatGirl: @GitaThanaMD presenting the In4M study during the digital technology scientific workshop. Solutions to study challenges in….
0
2
0
RT @FDAOncology: #ASH22: Dr. Kelly Norsworthy @KellyNorsworth2 presents an FDA Perspective on Challenges Posed by Inconsistent Classificati….
0
2
0
RT @FDAOncology: Save the Date! National Black Family Cancer Awareness Week is June 15-21, 2023. Note our new web page: .
0
5
0