RCNS MedChem Research Group
@keserulab
Followers
97
Following
8
Media
14
Statuses
90
Medicinal Chemistry Research Group at the HUN-REN Research Centre for Natural Sciences, headed by György M. Keserű. Also on Mastodon: @[email protected]
Budapest, Hungary
Joined April 2021
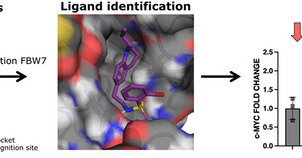
In collaboration with the group of @cgaldeano82, we have expanded the chemical space of E3 ligase binders by discovering new allosteric modulators of FBW7. Read the full article at
advanced.onlinelibrary.wiley.com
A computational approach to identify and characterize ligandable binding pockets on E3 ligases is presented. As a validation case, the first low-micromolar small moleculesthat bind to the FBW7 E3...
0
0
0
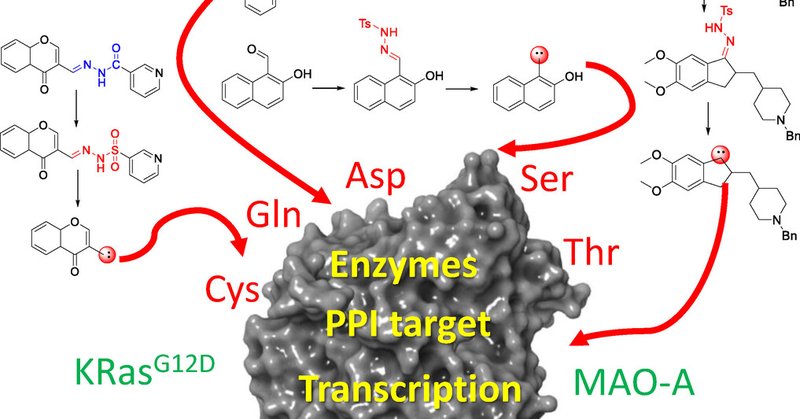
Let’s check the latest paper on covalent ligand efficiency from our group that extends the LE formalism to cysteine-targeting covalent ligands! Read the full article online:
0
0
2
The changing landscape of medicinal chemistry optimization https://t.co/RK6DbWHWkA This new article analyses oral drug candidates and their corresponding hit and lead compounds from recent years and from the early 2000s, and discusses the implications of the observed trends
0
25
108
Our paper on allosteric, covalent STAT inhibitors was highlighted in the "Recent article highlights" of @ACSMedChemLett.
pubs.acs.org
The STAT family of transcription factors are important signaling hubs, with several of them, particularly STAT3, being emerging oncotargets already investigated in clinical trials. The modular...
0
0
0
We are happy to welcome Tamás Dobai from the Institute of Parasitology in České Budějovice, Czechia, who will be working with us for the next months on the computational modeling of parasitic enzymes.
0
0
0
Rounding off an incredible streak, Levente Kollár is our third junior colleague to successfully defend his PhD thesis in the past two weeks, with full points from the on-site committee! Congratulations to all three of you, we’re super-proud! 👏
0
0
1
We congratulate our colleague, Dénes Szepesi Kovács, for his successful PhD defense! (Overwhelming support from the on-site Doctoral Committee, final decision by the Univ. Doctoral Board later this year.) 👏
0
0
0
We congratulate our colleague, Nikolett Péczka, for her successful PhD defense! 👏Full points from the on-site Doctoral Committee, final decision by the Univ. Doctoral Board later this year.
0
0
1
Our colleague, Dávid Bajusz, was appointed as a member of the Hungarian Young Academy:
0
0
1
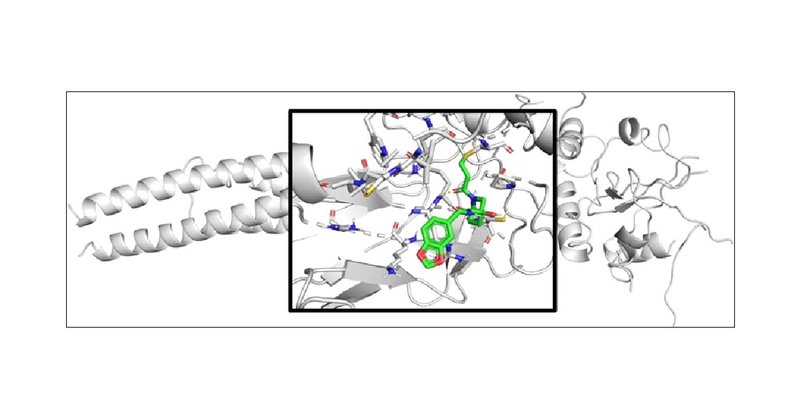
In our recent work, we have utilized a scarcely-studied #allosteric site of STAT3 to design new covalent inhibitors.
pubs.acs.org
The STAT family of transcription factors are important signaling hubs, with several of them, particularly STAT3, being emerging oncotargets already investigated in clinical trials. The modular...
0
0
1
Following our recent review on #fluorescent probes for #GPCRs, our latest paper details an interesting case study of the M2 receptor. https://t.co/cdG3zWEvLW
pubs.acs.org
The M2 muscarinic acetylcholine receptor (M2R) is a G protein-coupled receptor involved in regulating cardiovascular functions and mediation of central muscarinic effects, such as movement, tempera...
0
0
3
Our latest review in Eur J Pharm Sci summarizes recent advances (2018–2024) in #fluorescent probe development for Class A #GPCRs, analyzing over 120 newly developed probes covering 60 GPCRs.
0
1
2
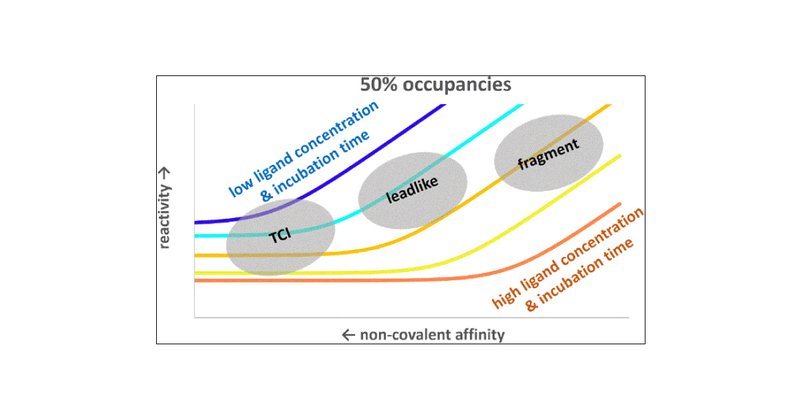
How do affinity and reactivity shape covalent target engagement? In our latest paper in J Med Chem, we show the distinct advantages of lead-like compounds vs. fragments in TCI design. #CovalentDrugs #MedicinalChemistry #DrugDiscovery
https://t.co/ZRx5TeygVf
pubs.acs.org
Labeling proteins with covalent ligands is finding increasing use in proteomics applications, including identifying nucleophilic residues amenable for labeling and in the development of targeted...
0
0
1
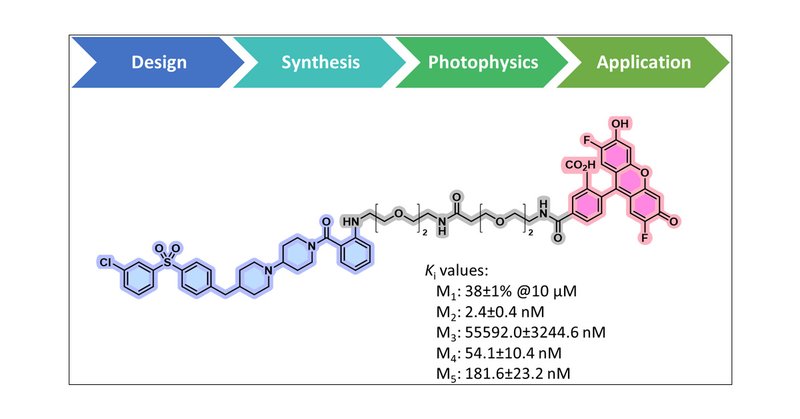
Our new photoaffinity labelling technology ( https://t.co/6LbhlQ8KEf) is published in @angew_chem. It is more biocompatible, more efficienct, cheaper and easily accessible, even as a late-stage modification. We are working with @EnamineLtd to make it available for the community.
onlinelibrary.wiley.com
Sulfonyl hydrazones were identified as new photo-crosslinkers exploiting their base catalyzed low-energy photoactivation that leads to reactive carbenes. The amino acid promiscuity, their usefulness...
0
1
3
We are happy to welcome Elisa Patacchini from the Sapienza University of Rome, who will be working with us for the next months on the computational modeling of GPCR ligands.
0
0
0
Our work on the benchmarking of uHTVS workflows against DEL libraries was featured on the cover of JCIM @JCIM_JCTC. https://t.co/cp6MnmnFk6
0
2
5
Our joint work with the Martinek group of @Uni_Szeged on local surface mimetics was featured on the cover of @angew_chem! https://t.co/3K7MD2a00W
0
1
3
Check out our latest paper on the role of the water network in protein-ligand binding. Fantastic collaboration with our colleagues from @pte1367 and Univ. Ljubljana. @janezilas
pubs.acs.org
Rational drug design focuses on the explanation and prediction of complex formation between therapeutic targets and small-molecule ligands. As a third and often overlooked interacting partner, water...
0
2
2
In the past three days, we have presented our activities to the curious visitors of the first Science Expo exhibition in the magnificent Museum of Fine Arts, Budapest. Thanks for our fantastic PhD students for their volunteer work!
niu.hu
Our goal is to make Hungary the birthplace of innovation, new inventions, start-ups and successful businesses.
0
0
1
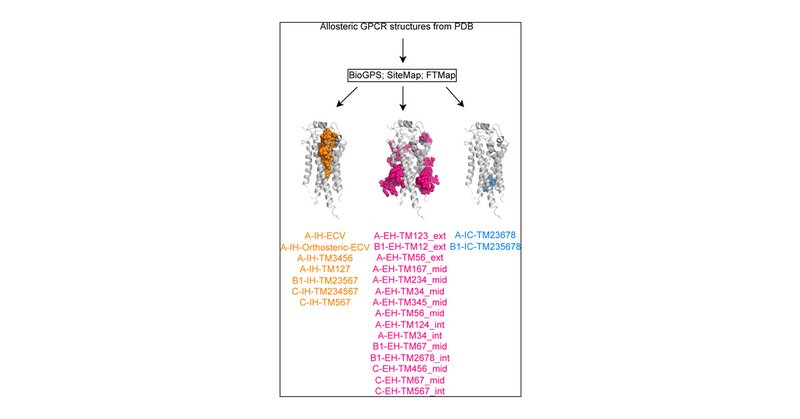
Check out our latest paper with @SonjaPeter4, @Chris_de_Graaf, @ALLODD_ITN, @NxeraPharma, on the systematic annotation and ligandability assessment of allosteric GPCR binding sites.
pubs.acs.org
The steadily growing number of experimental G-protein-coupled receptor (GPCR) structures has revealed diverse locations of allosteric modulation, and yet few drugs target them. This gap highlights...
0
2
1