Explore tweets tagged as #PipelineFlex
Embolización de Aneurisma Trigéminal Persistente con Stent #pipelineflex #Neurointervention #endovascularsurgery
0
0
1
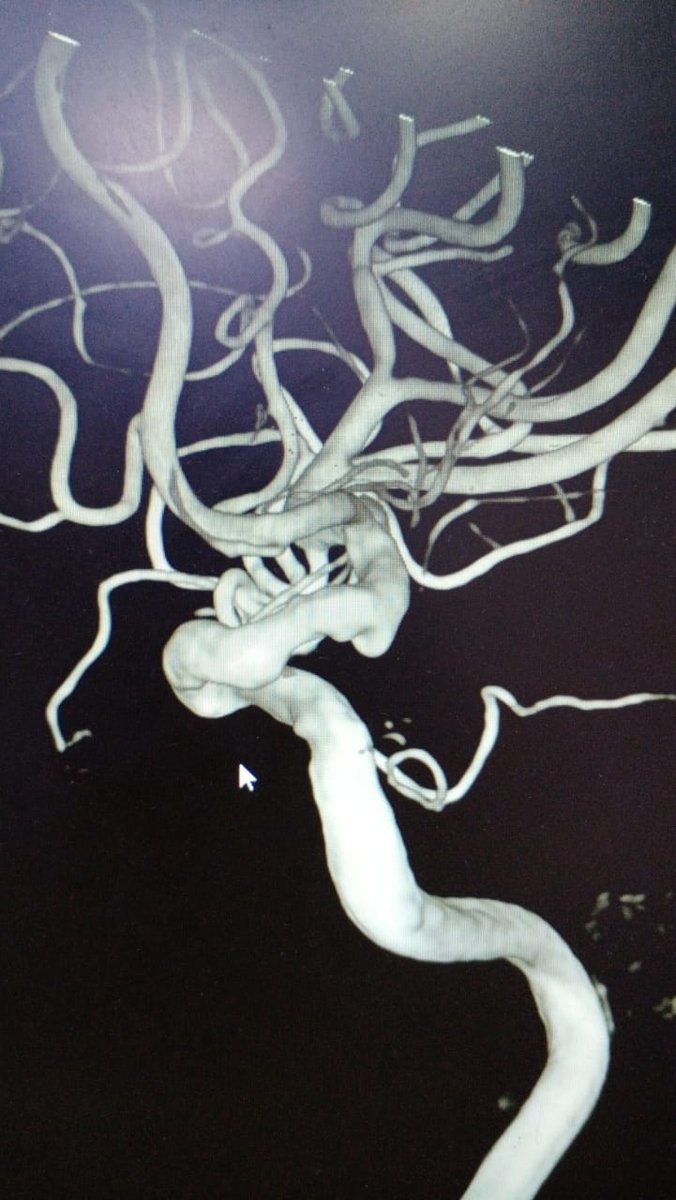
Ruptured right PCA dissecting #BrainAneurysm treated with 2 #PipelineFlex - immediate stabilization of saccular component. #RadialFirst #RadialForNeuro Benchmark > Phenom 027. #PipeIt #Neurosurgery #SpectrumNeuroscience
2
4
29
FDA: Pieces of this Recalled Device May Stay Inside a Patient’s Brain: #FDA #Drugwatcher #FDARecall #Medtronic #PipelineFlex
0
0
0
Although that recall did not include Pipeline Flex products with Shield Technology, Medtronic recalled 822 devices in the US. Read more 👉 #FDA #Drugwatcher #FDARecall #Medtronic #PipelineFlex
0
0
0
The recalled Pipeline Flex embolization device is a cylindrical permanent mesh stent used in treating certain intracranial aneurysms. Read more 👉 #FDA #Drugwatcher #FDARecall #Medtronic #PipelineFlex
0
0
0
The May 2018 cover illustration of @JVascSurg is Dr. Charles Matouk's treatment of a symptomatic, cervical ICA pseudoaneurysm that occurred a year ago with overlapping @Medtronic #pipelineflex devices. Great result and innovative strategy! @VascularSVS .
0
3
7
Some shots from our @Medtronic educational course hosted yesterday at @UnityHealthTO — live case, lectures, & expert discussion. A shout out to @vitorpereiracan and our fellows for a flawless case and to @ValRojas_TO for the organization. #NeuroIR #PipelineFlex #MedEd
1
2
8
FDA approves Medtronic's PipelineFlex embolization device: The US Food and Drug Administration (FDA) has grant. http://t.co/3i5u6jAtcb.
0
0
0
DID YOU KNOW? Our #Replicator pioneered research for the #PipelineFlex, a breakthrough flow diverter. Replication of pathology & procedure allowed professionals to get a better sense of the optimal methods & maneuvers required for successful outcome.
0
0
0
FDA approves Medtronic's PipelineFlex embolization device: The US Food and Drug Adminis. http://t.co/FcU2oXcLUc
http://t.co/BUS0Y5BVxP.
0
0
0
FDA approves Medtronic's PipelineFlex embolization device: The US Food and Drug Adminis. http://t.co/EhCcwTMXqn
http://t.co/rcLgeHlraw.
0
1
0