Ethan Hollingsworth
@ewholling
Followers
151
Following
491
Media
29
Statuses
98
MD/PhD Student in the Kvon Lab @UCIrvine | Gene regulation in development and disease
Irvine, CA
Joined August 2019
How do non-coding variants in enhancers cause human disease? Here, in my main PhD work with @evgenykvon, we uncover a surprising mechanism, with generalizable implications for human genomics. https://t.co/UHMAjxs6Qj n/
2
9
17
Awesome to see this story from our lab finally out! Shoutout to @gracecbower for making 4 new mouse lines just for revision experiments 🤯
Our preprint describing the Range Extender element, which is required and sufficient for long-range enhancer activation at the Shh locus, is out in @Nature. https://t.co/qFrjw55BtX
0
2
16
Also, a big thank you to @UCIBioSci and @NICHD for supporting this work. Link to paper:
0
0
2
So much of the credit goes to my mentor @evgenykvon for his investment & belief in this ambitious project. If any interested trainees are reading this, you would be hard-pressed to find a better environment to do science than in his lab.
1
0
2
Finally, they offer a mechanistic explanation for the excess of GOF variants observed in the literature. While LOF variants are buffered by shadow enhancers, another allele, and intra-motif redundancy, GOF variants lack such safeguards and instead are sensitized by poising.
1
0
1
Second, they provide a strategy for predicting the tissue-specific effects of noncoding variants. Geneticists can use snATAC-seq to identify tissues/cell types with open chromatin for a given locus, narrowing the large biological search space.
1
0
1
What are the broad implications of our findings? First, they suggest that pioneer TFs, not activators or repressors, confer cell-specific susceptibility.
1
0
1
To summarize: In some cells (pink), enhancers exist in a poised state. This poised state is conferred by pioneer TFs and sensitizes the cell type to ectopic activity by non-coding variants. We causally show this for the ZRS, and supporting evidence at previous and new loci.
1
0
0
This gave us an idea. 12/ Can we predict the in vivo effects of uncharacterized non-coding variants based solely on which tissues are poised? YES! We predicted and validated the forebrain-specific effects from non-coding variants identified in patients with autism.
1
0
1
Ok, what about other enhancers? 11/ We went back and looked at poising for other disease-linked enhancers. Every single enhancer became misactive in tissues that are poised, like this MYC enhancer linked to brain cancer.
1
0
1
What’s more, if we disrupt enhancer poising (via ZIC3 mutagenesis), we completely rescue severe polydactyly and tibial defects caused by patient variants. Without a poised enhancer, anterior cells are essentially de-sensitized to the effects of ZRS variants.
1
0
1
To answer this, we mutagenized the ZIC3 motif at the ZRS and looked at its effect on chromatin state. Strikingly, ZIC3 motif mutagenesis disrupted the poised enhancer signature, reducing open chromatin and K27ac in anterior cells.
1
0
2
This chromatin signature is reminiscent of a “poised” enhancer state. What underlies this poising signature? We performed RNA-seq on anterior vs. unaffected central cells and found ZIC3 to be upregulated, which binds to this highly conserved motif. So what is ZIC3 doing?
1
0
2
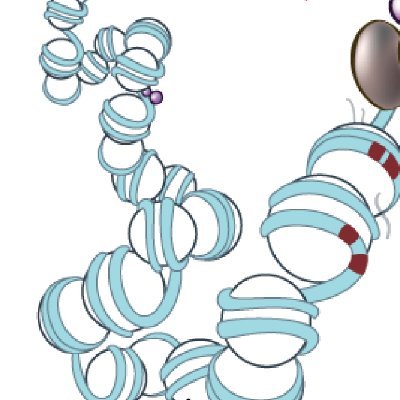
To answer this, we used a reporter mouse and FAC-sorted anterior cells to perform ATAC-seq & CUT&TAG for the WT ZRS locus. To our surprise, in anterior cells, the ZRS is accessible and marked by enhancer-associated modifications K4me1 and K27ac, despite not being active.
1
0
2
Using our dual reporter assay, we found that all ten independent variants cause ectopic activity in the same population of anterior cells. This suggests it’s not activators or repressors, but something else…
1
0
2
We dug into this contradiction by focusing on one example: the ZRS enhancer of Shh, at which gain-of-function (GOF) variants cause an anterior expansion of enhancer activity. So why anterior limb cells? Is it the type of variant (i.e., a disrupted repressor or activator)?
1
0
2
Many enhancer variants lead to ectopic gene activation across a variety of diseases like obesity, autism, cancer, limb malformations, etc. What’s puzzled us and others is although these variants are germline, they only cause misexpression in a handful of cells. Hmm….
1
0
2
Plasmid-based reporter assays are the bedrock of regulatory genomics. But a basic question has gone unanswered for decades: Do chromatin architectures form on plasmids transfected into mammalian cells—and does it matter? We finally have answers. https://t.co/34hcyRPN0z
biorxiv.org
Plasmids have fundamentally transformed how we resolve regulatory grammar across the tree of life. However, although chromatin plays an integral role in regulating the function of regulatory elements...
6
84
308
At 15, I left all my family and friends and came to the US , ALONE, seeking a country where MERIT and HARD WORK mattered-not politics. For 30 years, I worked tirelessly, even doing reseach at MIT the day my mother died, knowing she’d want me to keep pushing forward. Today, my
431
775
5K