The Magauer Lab
@MagauerGroup
Followers
3K
Following
611
Media
52
Statuses
139
Synthesis and Synthetic Methodology
Innsbruck, Austria
Joined March 2019
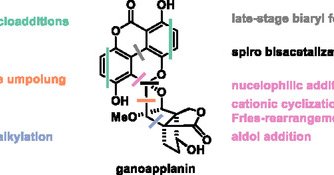
RT @ChemistryEur: Evolution of a Strategy for the #TotalSynthesis of the Ganoderma Meroterpenoid Ganoapplanin by Thomas Magauer and co-work….
chemistry-europe.onlinelibrary.wiley.com
Herein, the development of the first total synthesis of the Ganoderma meroterpenoid ganoapplanin is presented. Insights from unsuccessful approaches guide the design of an efficient route centered...
0
8
0
RT @OndrejKovac1: The final chapter of the Ganoapplanin story is here! Check out our team’s effort (@MagauerGroup ) toward this complex mer….
chemistry-europe.onlinelibrary.wiley.com
Herein, the development of the first total synthesis of the Ganoderma meroterpenoid ganoapplanin is presented. Insights from unsuccessful approaches guide the design of an efficient route centered...
0
3
0
RT @TotalSynthesis: #TotalSynthesis of Dactyloquinone A and Spiroetherone A via a Metal-Hydride Hydrogen Atom Transfer (MHAT) Process and a….
0
20
0
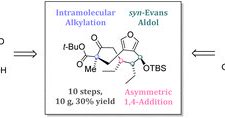
Congrats to Jan and Christian for their intriguing total synthesis of the maleidride natural products glauconic acid and glaucanic acid, achieved through an intramolecular alkylation strategy.
pubs.rsc.org
We disclose the first total synthesis of the maleidride natural products glauconic acid and glaucanic acid. The strategy relied on an early syn-Evans aldol reaction and an asymmetric 1,4-addition to...
1
3
43
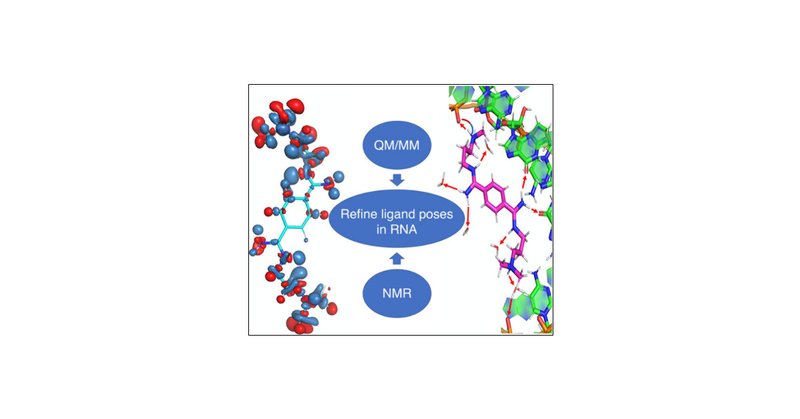
Also congratulation to our PhD student Aldo for his successfull collaboration with the Tollinger group, resulting in a publication in @JPhysChem .
pubs.acs.org
Predicting the binding poses of ligands targeting RNAs is challenging. Here, we propose that using first-principles quantum mechanics/molecular mechanics (QM/MM) simulations, which incorporate...
0
2
18
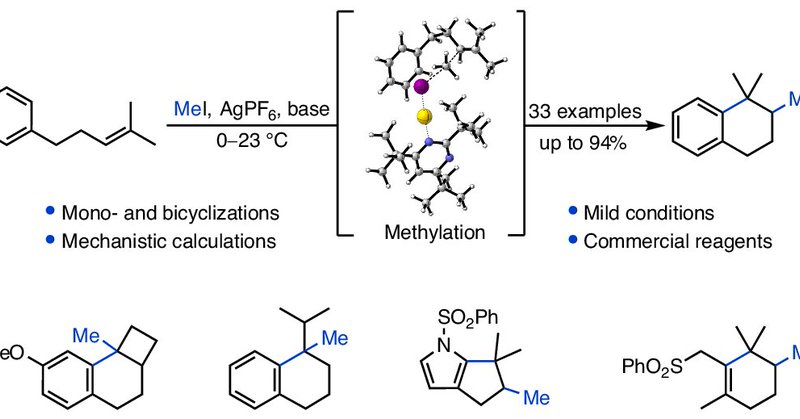
Our latest publication in @NatureChemistry describes a non-enzymatic methylcyclization of alkenes, mimicking the reactivity of Nature’s C-methyltransferases. Congrats to Immanuel, Elias and Sebastian! .
nature.com
Nature Chemistry - Bifunctional methyltransferase–cyclases both transfer a methyl group to alkenes and induce cyclization—a process called methylcyclization. Now a non-enzymatic...
3
8
99
And also congrats to Nico and Ondrej for the acceptance of their @synlett_journal paper about the "Development of a Triethylborane Mediated Giese Cyclization/Aldol Reaction Cascade for the Total Synthesis of Ganoapplanin".
0
3
14
Congrats to the Pimarane-Team for the acceptance of their article "Development of a Synthetic Platform for Ent‐Pimaranes Reveals their Potential as Novel Non‐redox Active Ferroptosis Inhibitors" in Chemistry - A European Journal (@ChemEurJ).
chemistry-europe.onlinelibrary.wiley.com
A full account of five distinct synthetic strategies towards a subfamily of ent-pimaranes is given. Highlights include radical and cationic polyene cyclizations for construction of the tricyclic...
0
7
65
Today, our department had the pleasure of hosting @KarlGademann for an inspiring presesentation on microbiome metabolites and engaging discussions. Grateful for the insights and perspectives!
0
0
23
Take a look at our latest work on the synthesis of ganoderma meroterpenoids published in @JOC_OL! Congratulations to Alex and Team Ganoderma!.
pubs.acs.org
Ganoderma meroterpenoids are fungal derived hybrid natural product class containing a 1,2,4-trisubstituted benzene ring and a polycyclic terpenoid part. The representatives applanatumol E, H and I,...
0
5
57
Last week, our PhD students Jan and Nico attended the 6th Boehringer Ingelheim MedChem PhD course. It was a great opportunity to gain insights into research in an industrial setting. Many thanks for the invitation @Boehringer
0
0
12
RT @OndrejKovac1: Ganoderma meroterpenoids strike back! Check out the latest contribution in @JOC_OL from @MagauerGroup. Congrats to Alex a….
pubs.acs.org
Ganoderma meroterpenoids are fungal derived hybrid natural product class containing a 1,2,4-trisubstituted benzene ring and a polycyclic terpenoid part. The representatives applanatumol E, H and I,...
0
3
0
Yesterday, we had the pleasure of welcoming @HeretschPhilipp to our institute, where he shared his exciting research on radical chemistry in natural product synthesis. It was a fantastic talk with engaging discussions! We really enjoyed having you with us.
0
0
12
Congratulations to Team Ganoderma on their successful publication of the synthesis of the meroterpenoid ganoapplanin!.
pubs.acs.org
The total synthesis of the Ganoderma meroterpenoid ganoapplanin, an inhibitor of T-type voltage-gated calcium channels, is reported. Our synthetic approach is based on the convergent coupling of a...
1
5
68
RT @TotalSynthesis: #TotalSynthesis of Ganoapplanin Enabled by a Radical Addition/Aldol Reaction Cascade by Nicolas Müller, Ondřej Kováč, A….
0
21
0