David Choi, PharmD
@IBDPharmD
Followers
402
Following
456
Media
31
Statuses
196
Clinical Pharmacy Specialist - Inflammatory Bowel Disease University of Chicago Medicine
Joined August 2021
The current prior authorization process leads to unnecessary delays that is detrimental to patient care! We need prior authorization reform now! @IBDMD @DocCohenIBD @UChicagoIBD @UChicagoGI @CrohnsColitisFn #IBD
academic.oup.com
Lay Summary. Despite a high approval rate, there were unnecessary delays in therapy due to prior authorizations. This study identified the impact of type o
2
14
51
Excited to have published our data on Skyrizi IV to subq transition (95%) at week 12! Barriers to transition 1) access/cost to therapy, 2) confusion of week 12 injection (pts thought it was 8 weeks after last IV loading dose) @IBDAPN @IBDMD #IBD #GITwitter.
accpjournals.onlinelibrary.wiley.com
There are no data evaluating the success rate in transitioning intravenous (IV) to subcutaneous (SQ) risankizumab-rzaa. To address these challenges, the University of Chicago Medicine (UCM) utilized...
0
1
20
With ease of starting IL23 for IBD (good insurance coverage, bridge program). Should we stop trying to dose escalate Stelara? There are substantial delays with appeals process (months) and extra work needed to appeal! #GITwitter #IBD.
1
0
2
Just kicked off our 3rd Annual IBD pharmacist symposium with @IBDProNews! We had a record 197 patients enroll with this symposium!! #IBD #gitwitter
2
3
24
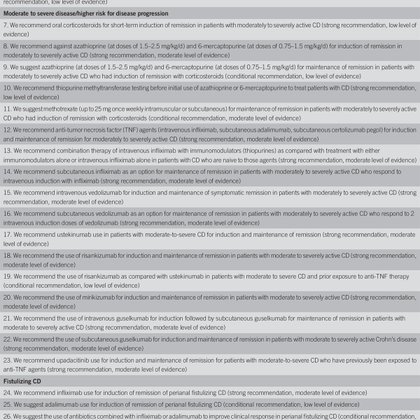
RT @EdwardLoftus2: ACG Clinical Guideline: Management of Crohn's Disease in. : Official journal of the American College of Gastroenterolo….
journals.lww.com
ideline was published. These guidelines represent the official practice recommendations of the American College of Gastroenterology and were developed under the auspices of the Practice Parameters...
0
37
0
RT @IBDPharmD: We are excited to be planning our third IBD Pharmacist Virtual Symposium for June 07, 2025! We are hoping to introduce Innov….
0
6
0
We have observed significantly higher rates (almost 30%) of acne with upadacitinib (JAKne) than in clinic trials (~7-8%). We found younger age, upadacitinib dose and female gender being risk factors for development! @amelia_kellar @IBDMD @Klein_Jeremy.
Grateful to be a part of this important work led by @IBDPharmD @IBDMD examining incidence and risk factors for development of acne in IBD patients treated with upadacitinib @Klein_Jeremy @UChicagoIBD.
1
8
32
One of the biggest challenges we have been encountering for #IBD care are the increasing role of quantity limit PAs! Although we are getting medications approved they are requiring a separate PA for the dose. These are leading to significant delays in care! #GITwitter.
0
3
9
Tremfya is FDA approved for Crohn's disease! This has both IV (200 mg) and subq (400 mg) induction loading dose options followed by subq maintenance dose (100 mg or 200 mg). #GITwitter #IBD .
jnj.com
TREMFYA® is the only IL-23i to demonstrate clinical remission and endoscopic response, both at one year, with a fully subcutaneous induction regimen Supported by data from the GALAXI study, TREMFYA®...
1
12
40
We are excited to be planning our third IBD Pharmacist Virtual Symposium for June 07, 2025! We are hoping to introduce Innovation Presentations that showcases amazing innovations by IBD pharmacy teams across the country! #GI #IBD #GITwitter.
🚨 Calling all #IBD #pharmacists! 🚨 Have an innovation that’s advancing IBD care? Submit your work for the IBD Pharmacist Virtual Symposium on June 7, 2025. 📝 Submission window: March 10 – April 4, 2025 .⏳ 15-minute presentations .Apply now! ➡️
0
6
19
IBD PharmDs can be an important member of the care team! This study shows delays in therapy if a PharmD is involved 🤔. The value an IBD PharmD brings ensures safe, effective, and timely initiation and continuation of therapies! #IBD #PharmD #GITwitter.
journals.lww.com
assessing insurance, care team, and patient-related factors. METHODS: Retrospective, multicenter study of patients with adult inflammatory bowel disease with prescriptions for an advanced therapy in...
3
4
25
Omvoh is now FDA approved for Crohn's disease! ##GITWITTER #IBD
investor.lilly.com
The Investor Relations website contains information about Eli Lilly and Company's business for stockholders, potential investors, and financial analysts.
1
2
4
RT @IBDAPN: 🚨 Today is the day! . Register here so you do not miss out on this incredible ChatAPP IBD Case Series by APPs for APPs! . https….
uchicago.zoom.us
Welcome! You are invited to join a meeting: ChatAPP: IBD Case series by APPs for APPS. After registering, you will receive a confirmation email about joining the meeting.
0
1
0
RT @ibdtweets: Clinical pharmacists should ideally be part of a multidisciplinary team to improve holistic care of IBD patients. 🌸 Clinical….
0
7
0
Please join us for this important discussion!! We will be discussing how to successfully start and keep patients on therapy and how to transition from IV to SQ successfully! #GITwitter #IBD.
Join us on January 14th at 6pm CST to participate in a dynamic discussion between @IBDAPN , @AngelinaIBDNP , #KimberlyOrleck & @IBDPharmD about sequencing, management and access to IL-23 therapies.
0
1
3
RT @IBDAPN: Join us on January 14th at 6pm CST to participate in a dynamic discussion between @IBDAPN , @AngelinaIBDNP , #KimberlyOrleck &….
0
1
0
The 7th Stelara biosimilar has been FDA approved! Ustekinumab-stba (Steqeyma; Celltrion) is expected to launch 02/2025! 🎉 #Biosimilar #IBD #GITwitter. -45 mg/0.5 mL or 90 mg/1 mL prefilled syringe.-130 mg/26 mL intravenous infusion.
pharmacytimes.com
Ustekinumab-stba (Steqeyma; Celltrion) is a subcutaneous treatment for adult and pediatric patients with plaque psoriasis arthritis, psoriatic arthritis, Crohn disease, and ulcerative colitis.
0
2
6
RT @GI_PharmD: 1️⃣st ever pharmacy track at #AIBD2024 ✅.Thanks to @MRegueiroMD @DrCoreySiegel @MLongMD @NMH_SHanauerMD @IBDConference for r….
0
11
0
What an amazing turnout to the first IBD Pharmacist Track at AIBD. @GI_PharmD and I are so grateful for the opportunity to have this session!! Thank you to AIBD for making this happens! #AIBD2024
4
6
38