Honggen Wang
@Honggen_W
Followers
710
Following
2K
Media
15
Statuses
116
Professor of synthetic organic chemistry at Sun Yat-Sen University, China
Joined October 2022
Finally published. Development of Indolo‐Bicyclo[3.1.1]Heptane as a Carbazole Isostere Through Radical Indolization of Bicyclo[1.1.0]Butanes - Liu - Angewandte Chemie International Edition - Wiley Online Library
onlinelibrary.wiley.com
The integration of two functional groups—isonitrile and alkene—into the Fukuyama radical indolization reaction of bicyclo[1.1.0]butanes (BCBs) has been successfully developed. This reaction offers a...
6
9
103
The trans-hydroiodination of alkyne is actually challenging. See our solution: Selective Synthesis of Z-α-Iodostyrenes via Hydroiodination and Photochemical Manganese-Catalyzed Geometric Isomerization | The Journal of Organic Chemistry
pubs.acs.org
Alkenyl iodides are essential intermediates in organic synthesis, traditionally synthesized via hydrometalation of alkynes or direct hydroiodination, which favor the formation of E isomers. However,...
1
4
48
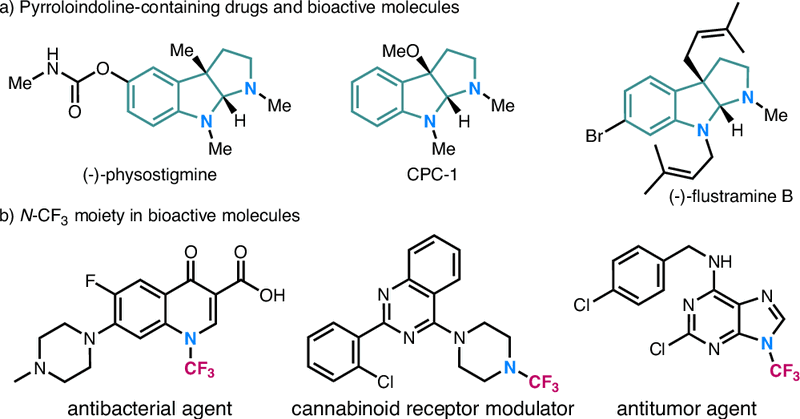
Our second work on the synthesis of N-CF3 compounds now out in Nat. Commun.. No column chromatography needed! https://t.co/tAkLAkVQwO
7
10
117
Our latest publication revealing a new β-boron effect is now out in Nature Communications: https://t.co/NB4CTGVhSr
2
14
108
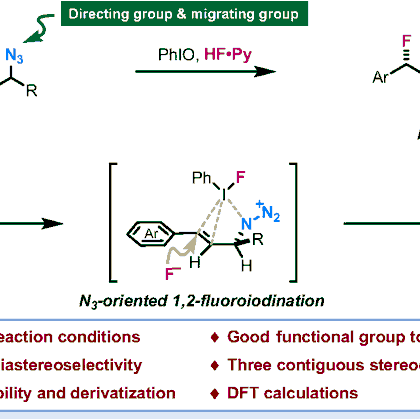
Our new publication: Dual Roles of Azido Group Enabled a Diastereoselective 1,3-Difluorination of Allylic Azides | CCS Chemistry
pubs.chemsoc.org.cn
1,3-Difluorinated molecules featuring fluorine-containing stereocenters often exhibit intriguing conformational properties attributed to 1,3-dipolar minimization effects. Here, we describe a diaste...
1
4
39
Our latest work on BCB chemistry presents a new route to indolo-bicyclo[3.1.1]heptane. Be sure to check out the beautiful work from Studer and Biju @ATBijuLab, using a different strategy: Studer: https://t.co/Wxkl3ZVXbM Biju: 10.26434/chemrxiv-2025-s0j6x https://t.co/ZzVjUuPCf0
9
9
105
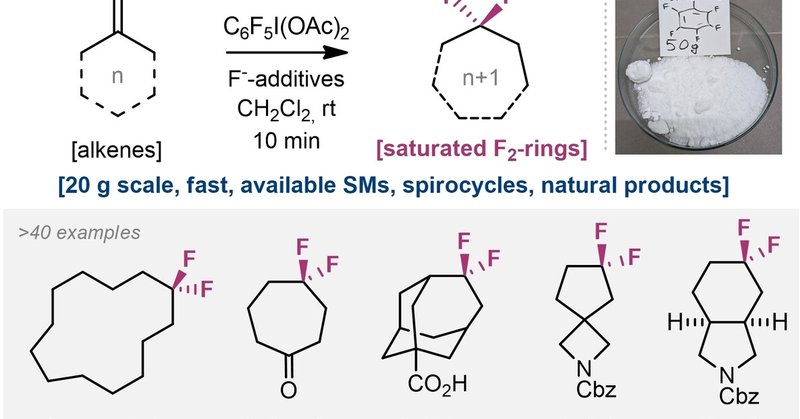
Saturated F2-Rings from Alkenes (Honggen Wang and co-workers) @mykhailiukche @Honggen_W •
onlinelibrary.wiley.com
Incorporating fluorine into aliphatic rings induces significant changes in molecular conformation, acidity/basicity, and lipophilicity. A general method has been developed to transform exocyclic...
0
12
89
@BarqQ @vadimscat The key reagent (EN300-46874786) is commercially available through @EnamineLtd! https://t.co/tLF2tqvHhp
0
5
23
With this magic chemistry, Ivan (@BarqQ) and Vadym (@vadimscat) have created some cool building blocks for medchem!
1
3
25
My favorite chemistry developed within our group—an incredible collaboration with Pavel @MykhailiukChem. Thrilled to see it finally published! Saturated F2‐rings from Alkenes - Wang - Angewandte Chemie International Edition - Wiley Online Library https://t.co/jlHDToMSqy
8
15
134
Just out @NatureCatalysis, our (retro) News & Views article on the birth of N-Heterocyclic Carbene Organocatalysis. Congratulations, @SukriyoC for the excellent summary👏! We thank the Editor, for the invitation to write this piece🙏! https://t.co/btHMcxTtKS
@iiscbangalore
30
14
236
Our new work: Stereodivergent atom transfer radical addition of α-functionalized alkyl iodides to alkynes: a strategy for selective synthesis of both E- and Z-iodoalkenes - now published in Chemical Communications
pubs.rsc.org
The geometrical control of atom transfer radical addition (ATRA) reactions to alkynes poses significant challenges. Herein, we present a uniform solution by developing a stereodivergent synthetic...
2
5
54
In collaboration with Pavel @MykhailiukChem, we have developed a general method for converting exocyclic alkenes into saturated F2-rings. Saturated F2-rings from Alkenes | ChemRxiv - https://t.co/uauRMjGI8Q
4
30
179
2-Azabicyclo[3.1.1]heptenes as potential bioisosteres for pyridines: Pyridine-boryl radical-catalyzed [3π 2σ] cycloaddition for the synth... https://t.co/sceyqmV00r
6
9
122
β-Silicon Effect Enables Metal-Free Site-Selective Intermolecular Allylic C–H Amination | ACS Catalysis https://t.co/gLlNRF3xzR Finally out in ACS Catal.. Many thanks to the students.
0
1
62
I am very happy to share that I’ve been recognised as one of the Royal Society of Chemistry’s 2023 outstanding reviewers. Explore the full list at https://t.co/BhvWCfvHsk
#RSCPeerReview
rsc.org
Celebrating some of the individuals making significant contributions to the chemical sciences through peer review
0
1
20
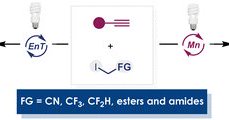
We are excited to share the publication of our latest work, "PolyBorylated Alkenes as Energy-Transfer Reactive Groups: Access to Multi-Borylated Cyclobutanes " in @angew_chem. This work, led by our heroes Nicol and @NadimEghbarieh. https://t.co/dmnQAR2VH3 Thanks to @angew_chem
17
12
110
A new application of beta-silicon effect. No desilylation occurs. beta-Silicon Effect Enables Metal-Free Site-Selective Intermolecular Allylic C−H Amination | ChemRxiv -
2
2
29
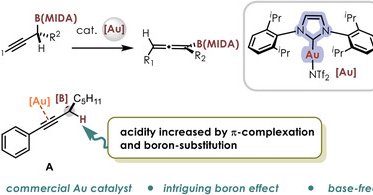
Our new publication reveals intriguing α-anion stabilization and α-cation destabilization of B(MIDA) moiety, leading to a NHC‐Au‐catalyzed base free isomerization of propargylic B(MIDA)s to allenes- Advanced Science - Wiley Online Library
advanced.onlinelibrary.wiley.com
An NHC-gold-catalyzed 1,3-H shift toward allenyl boronates synthesis from simple propargylic B(MIDA)s is reported. Mechanistic studies reveal dual roles of the boryl moiety in the reaction: to...
0
2
27