Crescendo Biologics
@HUMABODY
Followers
880
Following
68
Media
184
Statuses
258
Crescendo Biologics develops novel, targeted T cell enhancing Humabody® therapeutics. Leading its proprietary pipeline is CB307, a CD137 x PSMA bispecific.
Cambridge, England
Joined June 2015
Crescendo scientists and @Resolian_, have co-authored a paper in @BioanalysisJnl. The paper outlines a method to quantify CB307 (bispecific Humabody® VH) in blood, which is being used to support our ongoing Phase 1b POTENTIA trial.
tandfonline.com
Aim: Endogenous interferents can cause nonselectivity in ligand binding pharmacokinetic assays, leading to inaccurate quantification of drug concentrations. We describe the development of a Gyrolab...
1
0
1
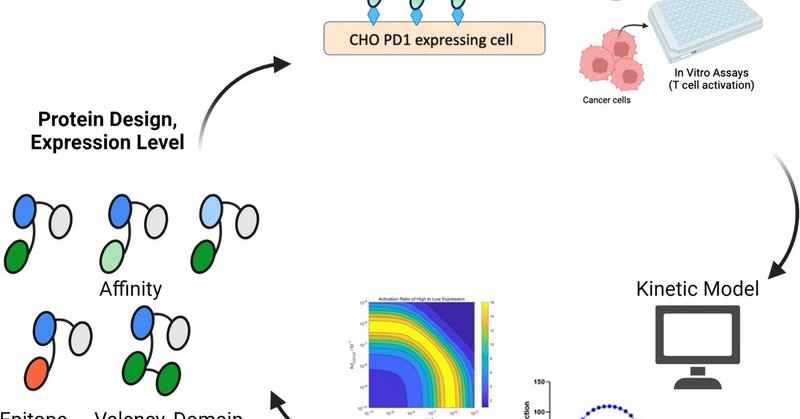
Our scientists along with Professor Greg Thurber’s laboratory at @UMichChE have co-written a paper recently published in The AAPS Journal, on the functional design of immune cell targeting therapeutics. #Biotech #Therapeutics #Discovery.
link.springer.com
The AAPS Journal - Bispecific and multispecific agents have become increasingly utilized in cancer treatment and immunotherapy, yet their complex design parameters present a challenge in developing...
0
1
2
We are excited to announce that our partner @ZaiLab_Global has dosed its first patient in a global Phase 2 clinical trial evaluating the efficacy and safety of the IL-17 targeted Humabody® VH ZL-1102, for the topical treatment of chronic plaque psoriasis.
1
0
1
Our CMO Kenji Hashimoto & CDO Philip Bland-Ward will be attending @ASCO from 31 May – 4 June in Chicago & look forward to connecting with #oncology professionals & hearing the latest innovations in #cancer. To arrange a meeting, contact: investors@crescendobiologics.com
0
0
0
Last night, Crescendo attended the @CambridgeIndy #Science and #Technology Awards. It is truly an honour to be a part of the #Cambridge scientific ecosystem. Thanks also to the sponsor of the ‘Biotech of the Year’ category @ChesterfordRP
0
0
2
We are thrilled to be publishing preclinical data for our lead programme, CB307, in Clinical Cancer Research, including our collaborative work with @ICR_London. (
0
1
3
We are delighted to have been named a finalist for 'Biotech of the Year', and for our CEO, Theo Harold, to have been nominated for 'CEO of the Year' at this year's @CambridgeIndy #SciTechAwards
0
0
1
We are pleased to be attending #BIOEuropeSpring 2024 in Barcelona. If you would like to meet us to discuss our Humabody® platform or T cell enhancing pipeline, please reach out to us via the partnering platform - we look forward to speaking with you. #biopharma #immunooncology
0
0
0
Our CEO Theo Harold and CDO Phil Bland-Ward are attending @TDCowen’s Health Care Conference from 4-6 March in Boston. To arrange a meeting with the team, contact: investors@crescendobiologics.com. #TDCowenHealthCare #Therapeutics
0
0
1
Our Chief Medical Officer, Kenji Hashimoto, joins the conversation in @PharmaScrip's latest #ScripAsks, about the therapeutic advances expected in 2024. To read the full article, please visit: #mCRPC #Cancer #Biotech #Therapeutics
0
0
0
Peer-reviewed journal Neoplasia has published a paper which details positive preclinical data for Crescendo’s half-life extended CD137 x PSMA, demonstrating superior tumour penetration. Collaboration with @UMichChE . PR: Paper:
crescendobiologics.com
0
1
4
We are delighted to have been included in @Labiotech_eu’s overview of innovative UK #biotech companies. Read @willowshahnev’s full article here: #immunotherapy #immunooncology.
labiotech.eu
Take a closer look at 21 of the top UK biotech companies, as the nation's biotech scene is now one of the most vibrant in Europe.
0
1
0
Our CMO Dr Kenji Hashimoto is looking forward to discussing the latest advancements in prostate cancer therapy and making new connections at the @ASCO #GU24 Cancers Symposium this week (25-27 Jan) #ProstateCancer #Immunotherapy
0
0
2
2023 has been an exciting and rewarding year for all of us here at Crescendo Biologics, bringing continued progress in the development of our lead asset CB307, a first-in-class programme for treating PSMA+ mCRPC. We look forward to another exciting year in 2024. #HappyNewYear
0
0
2
We are pleased to announce that the first patients in the US have been dosed in our ongoing Phase 1b study of CB307, Crescendo’s lead programme in PSMA+ metastatic castration-resistant prostate cancer (mCRPC). Read the press release here: @fredhutch
0
0
2
Our VP, Translational Biology Andrew Pierce will be presenting at @CamNTF’s event ‘Exploring immunology disease processes, disease segmentation and immune therapeutics’, hosted by @AstraZeneca, taking place on 4 Dec in Cambridge. You can sign up here:
0
0
2