Guillaume de Lartigue
@GdeLartigue
Followers
891
Following
803
Media
41
Statuses
438
Associate Professor, Monell Chemical Senses Center + UPenn. Interoceptive neuroscientist. Specific interest in feeding, obesity and gut- brain signaling. He/Him
Joined September 2017
RT @SinghArashdeep: New in @jclinicalinvest .We identified GLP-1 receptor therapy as a promising Bardet-Biedl Syndrome #BBS treatment. 🔬 We….
0
8
0
RT @pipethero: Delighted to highlight new work by Kelly Jameson and colleagues showing that select microbial metabolites in the small intes….
cell.com
Neuroscience; Microbiome
0
35
0
RT @SSIBsociety: 📢 SSIB 2025 Annual Meeting.📍 Oxford, UK | 🗓 July 28–Aug 1.🌟 5 Days of Science, Networking, & Fun!. Keynotes: Sadaf Farooqi….
0
6
0
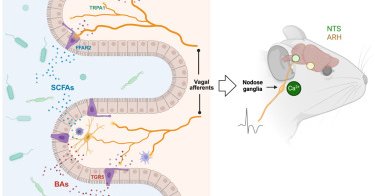
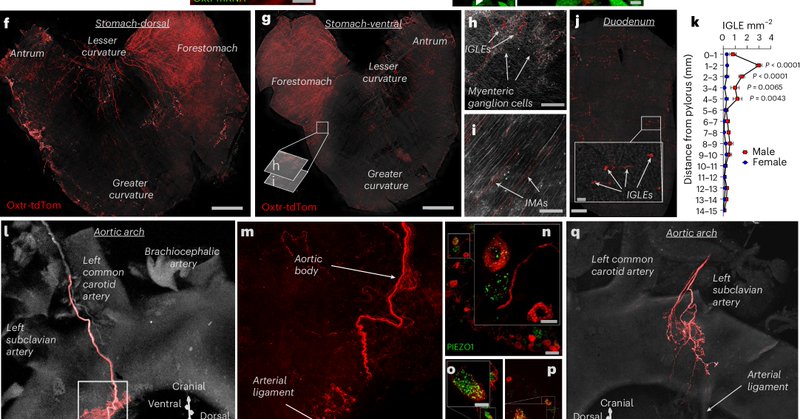
RT @egkrause_gsu: Excited by our latest joint production discovering body-to-brain communication that controls whole body metabolism. It wa….
nature.com
Nature Metabolism - Scott, Tan et al. characterize the neural circuits that link mechanic stimuli in the heart and gut with whole-body energy homeostasis and vigilance.
0
13
0
RT @zenbrainest: Chemogenetic excitation of NGOxtr creates patterns of brain activity associated with augmented hypothalamic–pituitary–adr….
nature.com
Nature Metabolism - Scott, Tan et al. characterize the neural circuits that link mechanic stimuli in the heart and gut with whole-body energy homeostasis and vigilance.
0
6
0
@mhrebecaaa @KElsaafien @javstern @scientistcaitb and twitterless Karen Scott, Dominique Johnson, Matthew Krichner, Sophia Eikenberry and Jessica Sa.
0
0
2
This work wouldn't have been possible without fellow corresponding authors @egkrause, and twitterless Annette deKloet. Huge thank you to all involved. We’d love to hear your thoughts! How do you see this being applied in the real world? Reply below! ⬇️.
1
0
2
Thrilled to share our second paper in two days, published today in #NatureMetabolism!. Yesterday, we explored food memory. Today, we reveal something entirely different: peripheral vagal circuits can induce a torpor-like state in mice. 🧵.
1
21
125
RT @MonellSc: 🧵 Can memory influence what and how much we eat?. Short answer is yes!. Memory is often overlooked as a key driver of food in….
0
7
0
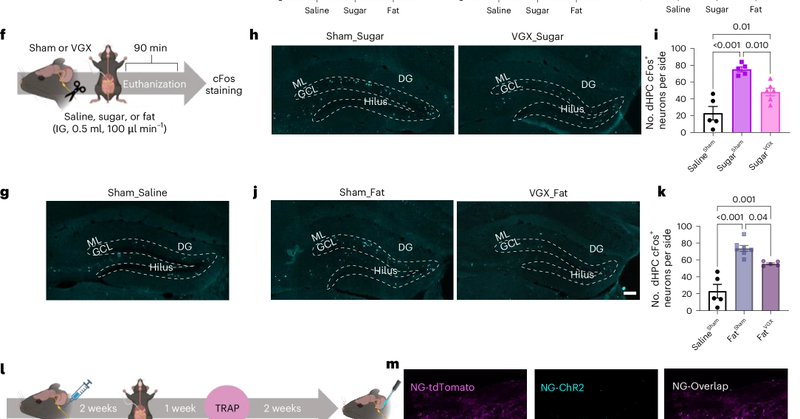
Read the full study here: �.And if you’d like to know more, feel free to reach out!.
nature.com
Nature Metabolism - Yang et al. identify and characterize distinct sugar- and fat-responsive neuronal populations in the dorsal hippocampus and their contribution to the development of obesity.
1
3
17
Huge thanks to our amazing team lead by superstar graduate student @mingxin_yang. This work was supported by @NIH and @American_Heart. Collaboration made it possible @MonellSc @USC! 🤝.
1
0
4