学術変革A-予知生合成科学
@ForecastBiosyn
Followers
349
Following
8
Media
23
Statuses
155
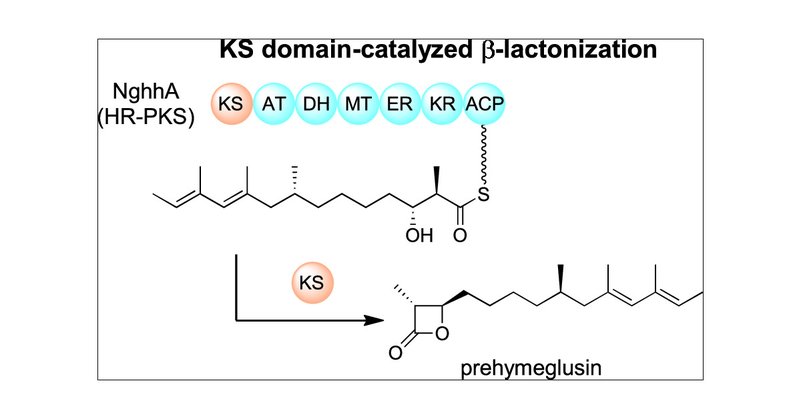
The Ozaki (A01) group, in collaboration with Prof. Teigo Asai group @Asai__lab, demonstrated that the ketosynthase domain of a highly-reducing polyketide synthase catalyzed β-lactone formation in the biosynthesis of hymeglusin. @J_A_C_S
pubs.acs.org
Hymeglusin (1) is a fungal polyketide consisting of a β-lactone ring with a unique (3R,4R) configuration. 1 is a potent 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) synthase inhibitor. Because it...
0
10
19
The Ushimaru group (A01), in collaboration with Prof. Hung-wen Liu, has uncovered a sulfur incorporation mechanism generating the thionucleoside antibiotic albomycin. @NatureCatalysis.
nature.com
Nature Catalysis - The mechanism by which sulfur is incorporated into the furanose ring in albomycins—a group of natural nucleoside antibiotics—remains unclear. Now, this work explains...
0
5
12
The Oguri group (A03) has established a synthetic strategy that enables scaffold-level redesign of ecteinascidins, unlocking access to potent anticancer macrocycles. available at: press release: @Chem_CP.
cell.com
A strategic redesign of ecteinascidin 743 scaffold, shifting the C1–C4 bridge to C1–C5, enables divergent access to 14- to 17-membered macrocycles with expanded three-dimensional architecture. The...
0
8
18
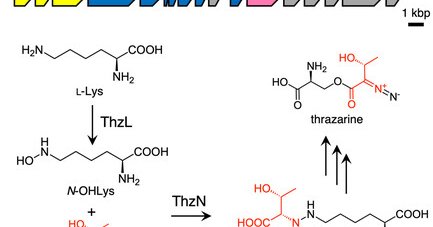
The Katsuyama group (A03) discovered the biosynthetic gene cluster for thrazarine, a diazo group-containing amino acid, in actinobacteria and the hydrazine synthase ThzN catalyzing formation of a key intermediate from L-threonine and N6-hydroxylysine.
chemistry-europe.onlinelibrary.wiley.com
A putative thrazarine biosynthetic gene cluster is discovered in the genome sequence of Streptomyces coerulescens MH802-fF5. In vivo and in vitro analyses of ThzN, a hydrazine synthetase with cupin...
0
7
11
RT @ykguitar1002000: Chemoenzymatic synthesis and in vitro selection of de novo thiazole‐containing macrocyclic peptides - Saito - Chemistr….
chemistry-europe.onlinelibrary.wiley.com
Backbone Thz moieties prevail in bioactive peptidic natural products and play important roles in their biological functions. Here, we report an in vitro selection platform for Thz-containing macroc...
0
1
0
The Goto group (A03), in collaboration with Prof. Onaka (A03), developed an in vitro selection platform for macrocyclic peptide ligands bearing backbone thiazoles via posttranslational chemoenzymatic conversion. @ChemEurJ.
chemistry-europe.onlinelibrary.wiley.com
Backbone Thz moieties prevail in bioactive peptidic natural products and play important roles in their biological functions. Here, we report an in vitro selection platform for Thz-containing macroc...
0
4
8
第六回公開シンポ���ウムは2025年7月18-19日に福井県繊協ビル 10 階ホール(福井駅徒歩10分)で開催いたします!参加登録を受け付け中ですので、奮ってご参加ください!
bio-4cast.skr.jp
2025年7月18-19日に開催する第六回公開シンポジウムの参加登録を開始しました。参加希望の方は以下のフォー
1
4
4
RT @J_A_C_S: Arginine-N,N′-bisprenyltransferases: Switchable Catalysis in Consecutive Guanidine-N-prenylation | Journal of the American Che….
pubs.acs.org
Lipidation is a promising strategy to enhance the membrane affinity and the serum stability of peptide drugs. Cyanobactin prenyltransferases catalyze the prenylation of peptides with diverse chemos...
0
5
0
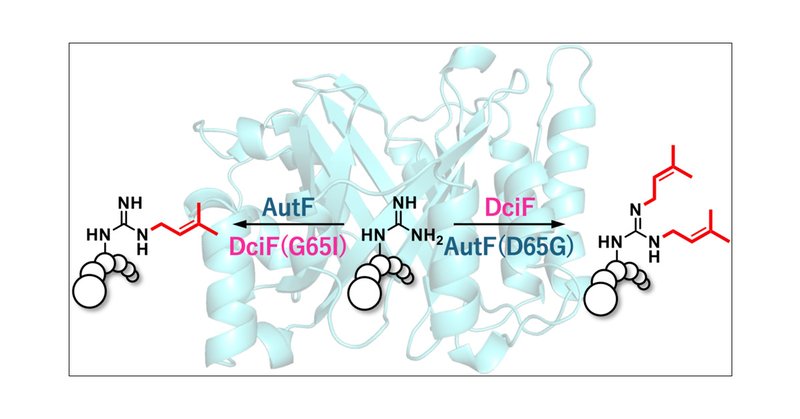
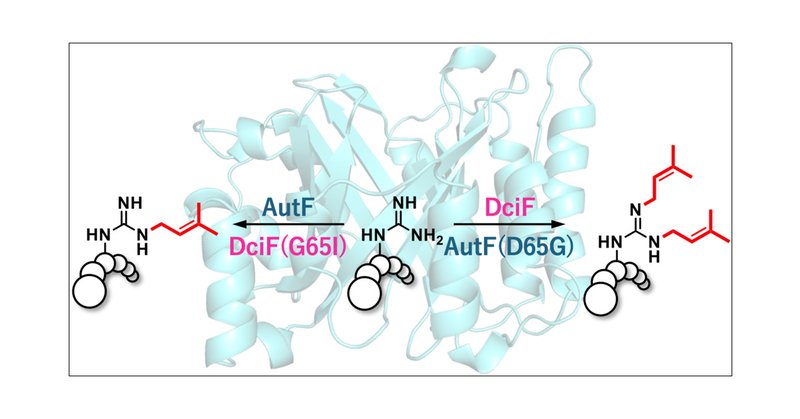
The Wakimoto group (A03), in collaboration with Prof. Ikuro Abe, has determined the crystal structure of a new, highly versatile Arg-N,N′-bisprenyltransferase DciF, unveiling the structural basis of its unique bisprenylation reaction. @J_A_C_S
pubs.acs.org
Lipidation is a promising strategy to enhance the membrane affinity and the serum stability of peptide drugs. Cyanobactin prenyltransferases catalyze the prenylation of peptides with diverse chemos...
0
10
19
RT @ykguitar1002000: Expanding the Diversity of the Terpene Skeleton and Structure through Identification of Noncanonical Class IE and IF T….
pubs.acs.org
Noncanonical class IE and IF terpene synthases have recently been discovered from bacterial genomes through the nonsequence-similarity-based method: protein-structural-model-based genome mining. This...
0
3
0
The Sato group (A01) demonstrated that the protein-structural-model-based genome mining can expand the diversity of terpene skeletons and structures.| Organic Letters.
pubs.acs.org
Noncanonical class IE and IF terpene synthases have recently been discovered from bacterial genomes through the nonsequence-similarity-based method: protein-structural-model-based genome mining. This...
0
8
15
The Katsuyama (A03) and Terada (A02) groups revealed the mechanism of ATP-dependent diazotase by combining structural biology, biochemistry and computational analysis. @angew_chem #Enzyme #biosynthesis.
onlinelibrary.wiley.com
Several diazotases have been reported in recent years, but the detailed catalytic mechanism is unclear. From X-ray crystallography and Cryo-EM analysis, we obtained the structure of a diazotase...
0
10
26
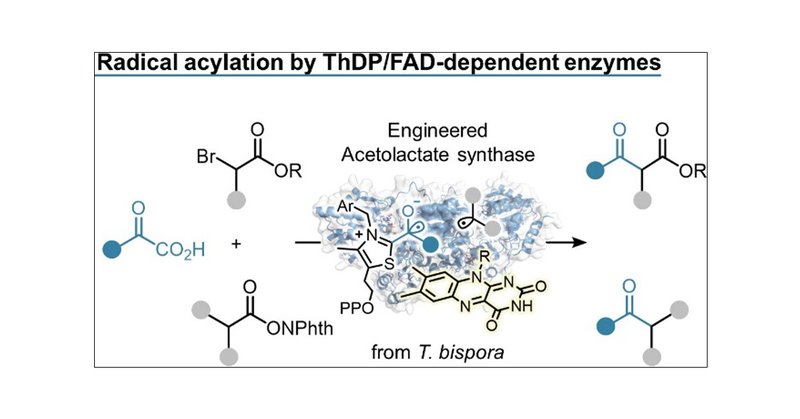
RT @J_A_C_S: NHC-Mediated Radical Acylation Catalyzed by Thiamine- and Flavin-Dependent Enzymes | Journal of the American Chemical Society….
pubs.acs.org
Cross-coupling reactions between short-lifetime radicals are challenging reactions in organic chemistry. Here, we report the development of an N-heterocyclic carbene (NHC)-mediated radical coupling...
0
10
0
The Kato group (A03) developed a novel biocatalytic system using T. bispora acetolactate synthase. The engineered variants showed high activity for abiotic radical acylation of α-bromo carbonyls and N-acyloxyphthalimides. @J_A_C_S.
pubs.acs.org
Cross-coupling reactions between short-lifetime radicals are challenging reactions in organic chemistry. Here, we report the development of an N-heterocyclic carbene (NHC)-mediated radical coupling...
0
7
21
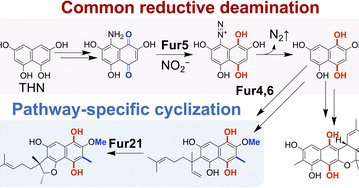
The Kuzuyama (A01) & Terada (A02) groups revealed the significance of the cryptic amino group of the hydroquinone intermediate common to the biosynthesis of tetrahydroxynaphthalene-derived meroterpenoids such as furaquinocin and naphterpin #MyFirstChemSci.
pubs.rsc.org
Hybrid isoprenoid-polyketides, known as meroterpenoids, are a family of natural products that exhibit various bioactivities and are promising drug scaffolds. Despite the structural diversity of...
0
10
17
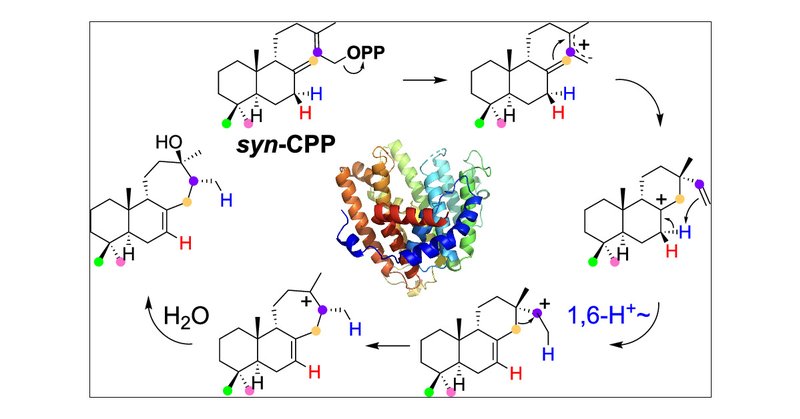
The Kuzuyama group (A01), in collaboration with Prof. Jeroen S. Dickschat, has identified a pair of cyanobacterial terpene synthases to produce a rare 6,6,7-tricyclic diterpene alcohol, deepening our mechanistic understanding of terpene cyclases.@J_A_C_S .
pubs.acs.org
In recent years, genome mining in cyanobacteria has revealed abundant gene clusters related to natural product biosynthesis. However, only a few terpene synthases (TSs) have been identified from this...
0
13
32