EBR Systems, Inc.
@EBRSystemsInc
Followers
373
Following
99
Media
132
Statuses
201
Leadless LV endocardial pacing for patients with heart failure.
Sunnyvale, CA
Joined September 2018
This Thanksgiving, we're grateful for the clinicians, partners, and patients who inspire progress every day. Thank you for your trust, collaboration and care. Wishing you a meaningful holiday filled with gratitude. #Thankful #Gratitude #PatientCare
0
0
2
Where does leadless LVEP fit into CRT pathways today? Join us on 11/22 at 5:30 pm ET at #RIO2025 to explore clinical rationale, patient selection, workflow integration & real case outcomes. Register: https://t.co/1QUmtZtZfM
0
0
0
Leadless LVEP is redefining CRT. Join a case review + live panel at #RIO2025 on 11/22 at 5:30 pm ET to explore endocardial rationale, workflow integration, outcomes & procedural sights. 📅 Register now: https://t.co/1QUmtZtZfM
0
1
2
Today we honor and thank all Veterans for their service, courage, and sacrifice. Your commitment inspires us every day. #VeteransDay
0
0
1
Congrats to @Drdevignair and team on completing the first WiSE-UP Study enrollments - advancing real-world evidence for the WiSE® System. Learn more: https://t.co/DHta76VnQK
0
1
6
New OUS studies explore investigational uses for the WiSE® System: 1️⃣ Totally Leadless CSP 2️⃣ CRT for non-responders These off-label applications aren't FDA-approved but point to future investigations. Read more: https://t.co/40KK159QTn eIFU & ISI: https://t.co/rESYPDpLMc
0
0
2
Big news from EBR: CMS has finalized NTAP and TPT reimbursement for the WiSE System, effective Oct 1, 2025 — marking a major step toward expanding access to leadless LV endocardial pacing (LVEP) in the U.S. 🔗 Learn more: https://t.co/2LmBaU3rHI eIFU: https://t.co/rESYPDpLMc
0
0
2
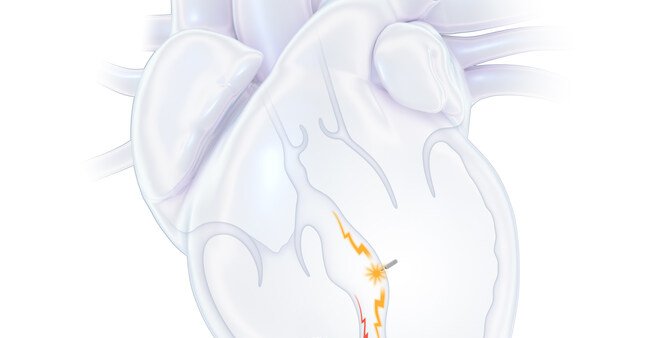
Here's some cool science for you: A device the size of a grain of rice that can help your heart beat in sync with your pacemaker. https://t.co/kENVZzUgKG
0
2
3
65-year-old Tom Derrington was running out of options. After receiving the WiSE System at St. David’s TCAI, he’s back to walking trails & even light construction work. Read his inspiring story: https://t.co/fdHMQc9Arw ISI:
ebrsystemsinc.com
EBR aims for outstanding treatment of cardiac rhythm diseases with an innovative leadless therapy.
1
0
1
Innovation in cardiac care changes lives. Watch how WiSE transformed David’s life in Naples, FL.: https://t.co/rYTRHiGqRL View approved indications & ISI:
ebrsystemsinc.com
EBR aims for outstanding treatment of cardiac rhythm diseases with an innovative leadless therapy.
0
0
1
Congratulations to Fogarty company-in-residence, @Materna_Medical on the completion of a recent close within an ongoing $20 million Series B2 round & the new commercial name for its second product, Ellora™ (formerly Materna Prep)! More: https://t.co/t7fq3lPq6n
0
2
4
Vital Signs Podcast: @EBRSystemsInc major milestones in WiSE CRT commercialisation https://t.co/ZTbpy9QGii
#ASX #Medtech $EBR
0
1
3
Vital Signs Podcast: EBR’s major milestones in WiSE CRT commercialisation
stockhead.com.au
In the episode, we unpack EBR Systems' (ASX:EBR) major milestones this year and next steps in the commercial roll out of its tech.
2
3
12
First U.S. commercial implants of the FDA-approved WiSE System are complete—thanks to 🫀 Dr. Robert Canby + team at @StDavidsHC 🫀 Dr. Niraj Varma @ClevelandClinic Leadless LVEP is here for heart failure patients who had no other options. Press Release:
prnewswire.com
/PRNewswire/ -- In a major milestone for heart failure treatment, the first commercial patients in the U.S. have been successfully implanted with the...
1
2
18
Break it Down: @EBRSystemsInc completes first US commercial implants of WiSE CRT system. Watch the video here: https://t.co/P8ZkT0FSbv
#ASX #Medtech $EBR
0
1
3
Please share this opportunity with colleagues and friends! The Fogarty Prize recognizes an individual or team who has developed and commercialized a singular medical technology that has meaningfully advanced patient care. #medtech #innovation #impact
0
1
3