Csaba Pal Laboratory
@Csaba_Pal_Lab
Followers
593
Following
224
Media
13
Statuses
173
The lab's interest includes antibiotic resistance, systems biology, and microbial experimental evolution. Account run by lab members. @csabapallab.bsky.social
Szeged, Hungary
Joined September 2019
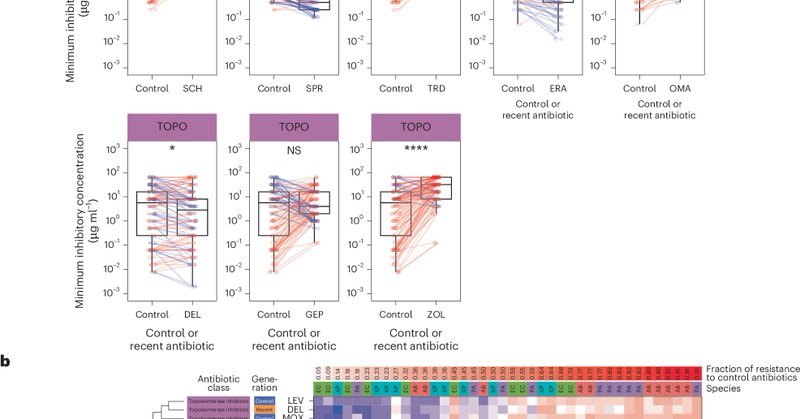
🚨 Our latest research in @NatureComms shows a promising strategy for less resistance-prone #antibiotics. For details, see the thread below and read the paper “Exploring the principles behind antibiotics with limited resistance” here:
nature.com
Nature Communications - This study shows that only those dual-targeting antibiotics limit resistance in Gram-negative pathogens that also target the membrane of the bacteria. This mechanism...
1
10
27
@Ely_win @marczikkely @BiochemBrc @BiologicalRese1 For previous research on #FutureAntibiotics and resistance development see our recent publications here: and
nature.com
Nature Microbiology - An extensive experimental analysis of resistance to antibiotics in development or introduced post-2017 in ESKAPE bacteria reveals the dynamics of resistance acquisition,...
0
0
3
5/5 - Contributors:.This work was spearheaded by @Ely_win and @marczikkely at @BiochemBrc @BiologicalRese1.
1
0
0
4/5 - Implications for the Future:.These insights provide a blueprint for developing next-gen antibiotics (#FutureAntibiotics) designed to stay one step ahead of bacterial defenses. It’s a promising direction in the fight against #AntibioticResistance #AMR.
1
0
0
3/5 - A stark contrast:.Dual-target topoisomerase antibiotics, despite having two intracellular targets, were more prone to resistance. This highlights that which targets matter just as much as how many. #DrugDevelopment.
1
0
1
2/5 – Dual target permeabilizers:.We tested three promising #DrugCandidates – POL7306, Tridecaptin M152-P3, and SCH79797 – against tough #ESKAPE pathogens like E. coli, K. pneumoniae, A. baumannii, and P. aeruginosa. The results? #ResistanceEvolution was significantly limited.
1
0
1
1/5 - The strategy: .By combining membrane disruption with another key cellular pathway, we can dramatically limit bacterial #resistance. This “double whammy” makes it much harder for bacteria to fight back. #RationalDesign #Antibiotics #AMR.
1
0
1
RT @hun_ren01: Antibiotics of the future are prone to bacterial resistance. #HUNREN #SZBK #BRC @BiologicalRese1 htt….
0
4
0
RT @KintsesLab: Excited to share a new collaboration with the @Csaba_Pal_Lab, out now in @NatureMicrobiol!.The study highlights how ESKAPE….
0
3
0
RT @CatiaCilloniz: New study reveals the rapid emergence of #antibiotic #resistance in ESKAPE pathogens. Resistance can develop in just 60….
0
25
0
RT @balazs_papp_lab: ESKAPE pathogens are outsmarting us. Our latest paper in @NatureMicrobiol reveals alarming ease of resistance evolutio….
0
3
0
RT @ScienceTM: The common bacterial pathogen #Saureus quickly evolves resistance to a range of experimental and recently approved #antibiot….
0
7
0
For a similar study on Gram-positive bacteria, check out our previous thread:
Thrilled to share our latest work, "Antibiotic candidates for Gram-positive bacterial infections induce multidrug resistance" in @ScienceTM! For details, see the thread below and read the paper here:
1
0
2
7/7 🔗 This study was spearheaded by @DarukaLejla, @marczikkely, @SziliPetra and @ZoltanFarkas81 at @BiochemBrc @BiologicalRese1. Important contributions from our long-term collaborators @balazs_papp_lab and @KintsesLab.
1
0
4
6/7 📢 Stay tuned! Our upcoming study will reveal a promising strategy to develop less resistance-prone antibiotics! #DrugDevelopment.
1
0
2
5/7 🔬 The study suggests that certain bacteria were less prone to develop resistance against some of the tested antibiotics, highlighting the potential in narrow-spectrum therapeutics. This presents hope for more effective future treatments. #Antibiotics #PublicHealth.
1
0
1
4/7 🌍 Functional metagenomics revealed that mobile resistance genes exist not only in clinical isolates but also in the soil and even the human gut microbiome. Resistance is EVERYWHERE, making it harder to tackle with new treatments. #GlobalHealth.
1
0
1
3/7 🧬 Even more worrying: resistance mutations were already present in natural bacterial populations. This means that some bacteria may already have the arsenal to resist future antibiotics they have never encountered. #EvolutionOfResistance.
1
0
1
2/7 🦠 We tested 13 new antibiotics (some on the market for a couple of years, others still in development) against 4 of the most critical Gram-negative pathogens. The result? Resistance emerged in just 60 days. This shows how quickly bacteria can adapt! #AntibioticResistance.
1
0
1
1/7 The rise of drug-resistant infections is a global crisis, but many pharma companies have abandoned antibiotic R&D. While some new drugs are in development, our study shows resistance is emerging rapidly, even to these #FutureAntibiotics. Let’s unpack the results.⬇️.
1
0
2