@onc_ce
@onc_ce
Followers
525
Following
417
Media
826
Statuses
2K
Welcome to your only source for accredited, serialized Tweetorials in the oncology space. Follow us for FREE CE/CME by expert authors!
Joined December 2021
Don't miss the launch of our new #accredited feed for 🆓 CE/#CME on #obesity management! A worthy companion to @onc_ce!.Launched today from @obesity_ce. FOLLOW US NOW!. XOXO, @academiccme
0
0
0
Congratulations to @BritSocHaem for their excellent new #CAT guidelines. Very practical, well-referenced. Check them out at @cardiomet_ce @aakonc @connors_md @TzufeiWang.@MarcCarrier1 @DrRohitMoudgil
0
2
12
13) And that's MY picks from among the many #FL highlights from #ASH23. Go forth now and claim your 0.5hr 🆓 CE/#CME at and FOLLOW US here at @onc_ce for more #hemonc #MedEd! Thanks to @BrianHill_MDPhD. #lymphomasm #OncTwitter.
oncologytweetorials-ce.com
1b) Don't miss @BrianHill_MDPhD's companion update from #ASH on mantle cell #lymphoma #MCL, posted this morning starting at https://t.co/RmzdEemHX ...
0
1
1
12b) It was c, #lisocabtagene maraleucel (#liso_cel), in the #TRANSCEND_FL trial presented at #ASH23. Now, which drug combined with #mosunetuzumab showed 89% #CR and 92% #ORR for 1L management of #FL?.
1
1
1
10a) And we saw f/u data from the original #mosunetuzumab study at #ASH23. Prior results of Ph 2 study (NCT02500407) ➡️ high #CR rate with manageable tox in pts w/ R/R #FL & ≥2 prior lines of therapy. See
1
0
1
9b) This study suggests high efficacy of #mosunetuzumab SC in 1L for these patients.👉 Fixed duration.👉#ORR of 96%, with 76% achieving #CR.👉Manageable toxicity profile.👉Notable: 3 CD20neg relapses.🗓️ Longer follow-up needed
1
0
1
9a) While we are talking about newly diagnosed #FL, #ASH23 also brought us new data for pts with newly diagnosed high-burden FL: with a subcutaneous formulation of the #CD20xCD3 #bispecific antibody #mosunetuzumab, by @falchi_lorenzo of @MSKCancerCenter.
1
0
1
8a) Meanwhile, in other #ASH23 news about #FL, the ubiquitous Prof Morschhauser also presented initial data from a Ph Ib/II trial for pts with newly dx'd FL tx'd with #mosunetuzumab in combo with #lenalidomide.
1
0
1
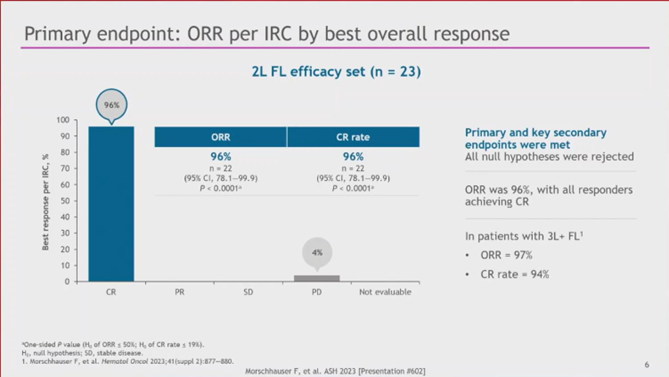
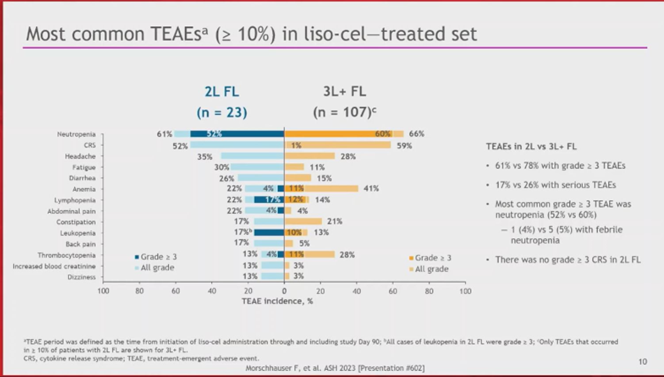
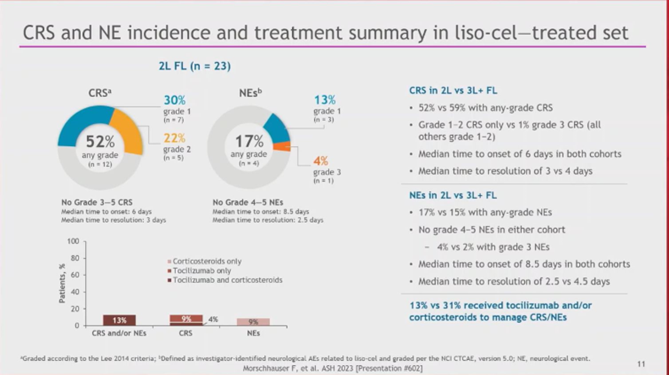
7d) Franck #Morschhauser, University of Lille, presented the primary analysis. #ORR & #CR were 96%, and the safety profile was favorable with low rates of severe (Gr ≥ 3) #CRS, #NEs, prolonged #cytopenia, and no severe infections. #lymphoma #lymsm #leusm
1
0
1
7c) These were high-risk pts, required to have dz progression within 24mos following tx received no longer than 6mos after original dx, high tumor burden as defined by #mGELF criteria, or both. All pts had received 1 prior combo of systemic tx + anti-CD20 antibody & alkylator.
1
0
1
7b) #TRANSCEND-FL (NCT04245839) is a global, Ph 2, single-arm, multicohort, pivotal study assessed efficacy & safety of the anti-#CD19 #CAR_T cell therapy #lisocabtagene maraleucel (#liso_cel) as 2L therapy in 26 pts with R/R #FL.
1
0
1
7a) Further on the #CAR_T front, we had new data presented at #ASH23: reported results of #TRANSCEND_FL.
1
0
1
6d) Another immunotherapeutic treatment option for R/R #FL is with chimeric antigen receptor T cells (#CAR_T). Specifically, #axicabtagene ciloleucel (axi-cel) is #FDA-approved for this indication, based on results of the #ZUMA-5 trial. See
pubmed.ncbi.nlm.nih.gov
Kite, a Gilead Company.
1
0
1