Explore tweets tagged as #Checkmate8HW
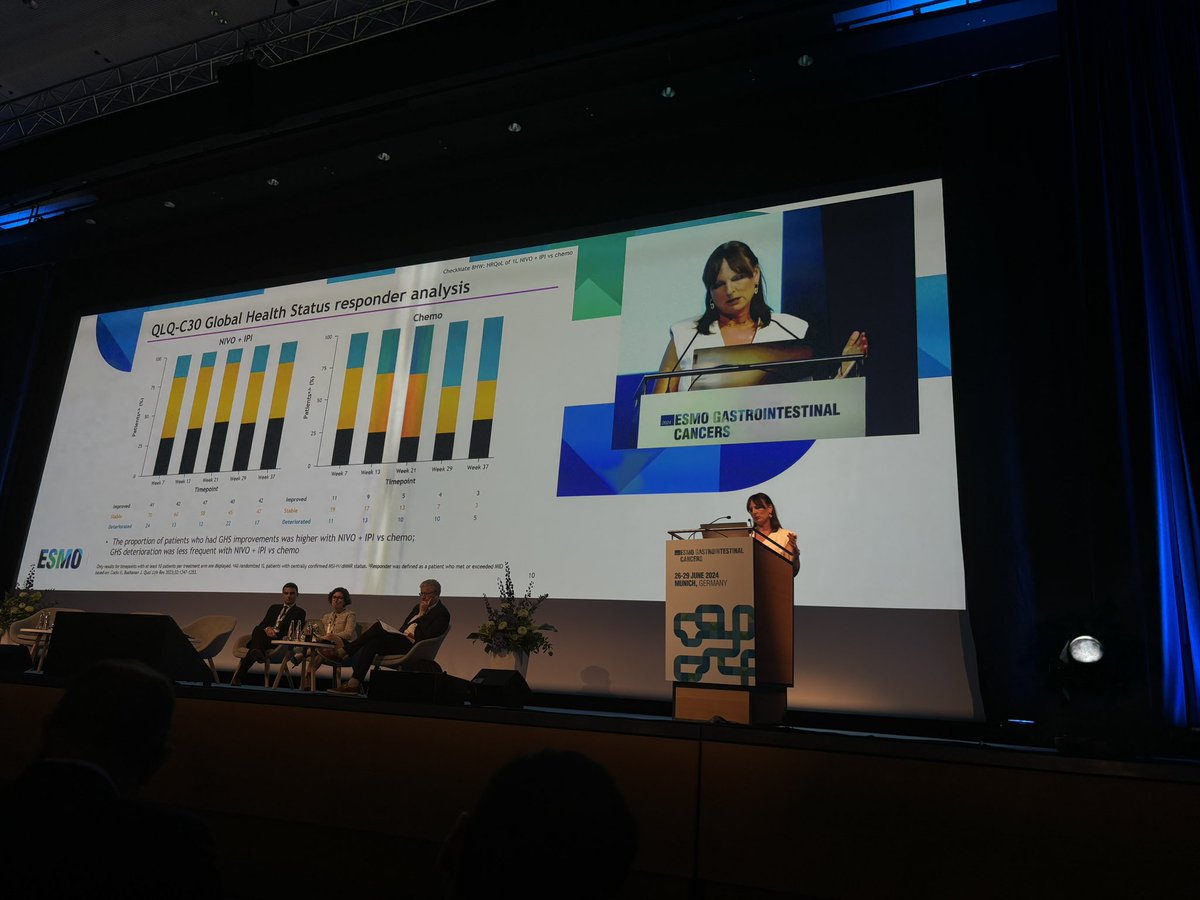
According to results from the #CheckMate8HW trial, #nivolumab plus #ipilimumab significantly improved health-related quality of life compared to nivolumab alone among patients with MSI-H/dMMR mCRC. Learn more: #medtwitter #onctwitter
0
0
0
#ESMOGI25 | Dr. Elena Élez shares insights with @ecancer on the #CheckMate8HW trial 🔬. 🎯 This study compares nivolumab + ipilimumab versus nivolumab alone in patients with MSI-H/dMMR metastatic #ColorectalCancer. Watch the video here 👇.
0
2
8
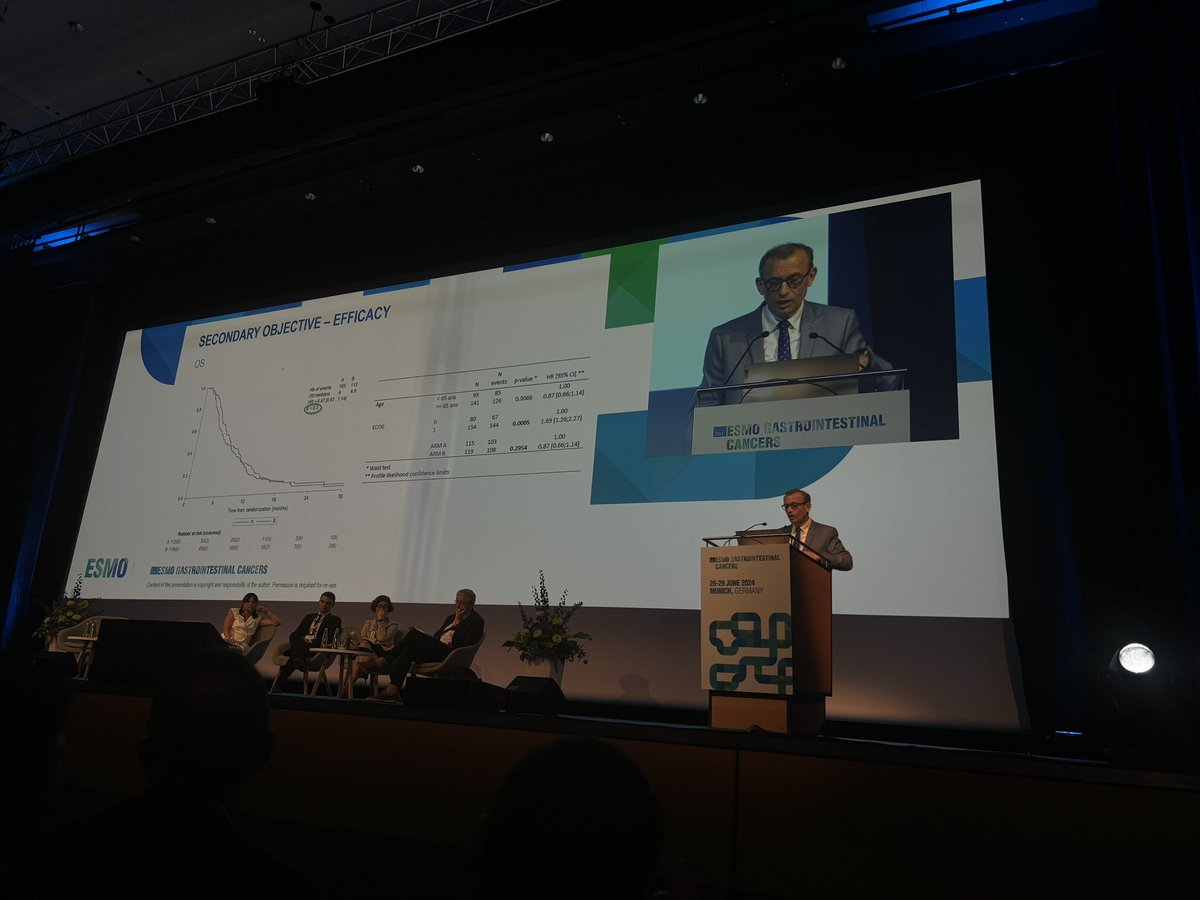
3. #CheckMate8HW: Ph 3, dMMR/MSI-H unresectable or mCRC, Ipi (1mg/kg) + Nivo (240mg) Q3W - 4 cycles and then Nivo alone vs Nivo vs chemo. - mPFS 54.1 for Ipi/Nivo vs 5.9 mos with chemo .- Early drop off with Nivo alone (vs Ipi/Nivo).- ORR: 71% (Ipi/Nivo) vs 58% (Nivo). 4/6
Ipi + Nivo is now approved in dMMR/MSI-H mCRC based off #CM8HW! Before #ASCO25, we had a chance to 🗣️ this trial, findings, dosing, single vs. dual ICI and AEs w/ @GIMedOnc . Full 🗣️:.⭐️ ⭐️ Also on “Oncology Brothers” podcast . #OncTwitter #gism #GiOnc
1
4
17
Ipi/Nivo now @FDAOncology approved based off #Checkmate8HW: Ph 3, dMMR/MSI-H mCRC, Ipi (1mg/kg) + Nivo (240mg) Q3W for 4 cycles and then Nivo alone vs Nivo:. - mPFS NR for Ipi/Nivo vs 39·3 months.- ORR: 71% vs 58%.- Any grade AEs 81% from Ipi/Nivo vs 71% Nivo alone. #OncTwitter
3
38
103
#ASCO2025-Updated #CheckMate8HW Results: Nivolumab + Ipilimumab Shows Sustained PFS Benefit for MSI-H/dMMR Metastatic CRC
0
0
2
Great presentations during the Proffered Paper Session at ESMO GI 2024 ✨.💫 Safety results of TRANSMET.💫 HRQoL data of CheckMate8HW.💫 Trifluridine-Tipiracil and Regorafenib endless battle: new warriors unlocked . And outstanding discussion by @ChiaraCrem1 ❤️ . @myESMO
2
4
13
ESMO GI 2025 Highlights: CheckMate 8HW Trial on HRQoL in MSI-H/dMMR mCRC with NIVO + IPI.@myESMO. #CheckMate8HW #ESMO2025 #Ipilimumab #Nivolumab #MetastaticColorectalCancer #OncoDaily #Oncology #Health #MedEd #MedX #Medicine
0
0
3
Our #GICancers Symposium roundtable discussions are now available on demand. Watch for expert reviews of top abstracts from San Francisco: #SKYSCRAPER08, #NETTER02, #EMERALD1 #COBRA #CheckMate8HW . 🎥 @scottrberry @GillSharlene #ProfMarcPeeters. #MedOnc
1
3
3
CheckMate 8HW: Dual Immunotherapy Improves Outcomes in MSI-H/dMMR mCRC. #ColorectalCancer #MSIH #dMMR #CheckMate8HW #Immunotherapy #CancerResearch #Nivolumab #Ipilimumab #GIOncology #ASCO25
0
0
0
In this week's Clinic Conversation, Christopher Lieu, of @CUCancerCenter, explores the #CheckMate8HW study, which investigated #immunotherapy in MSI-H or dMMR metastatic #ColorectalCancer, and how to discuss this with patients in the #clinic. Read here:
0
0
1
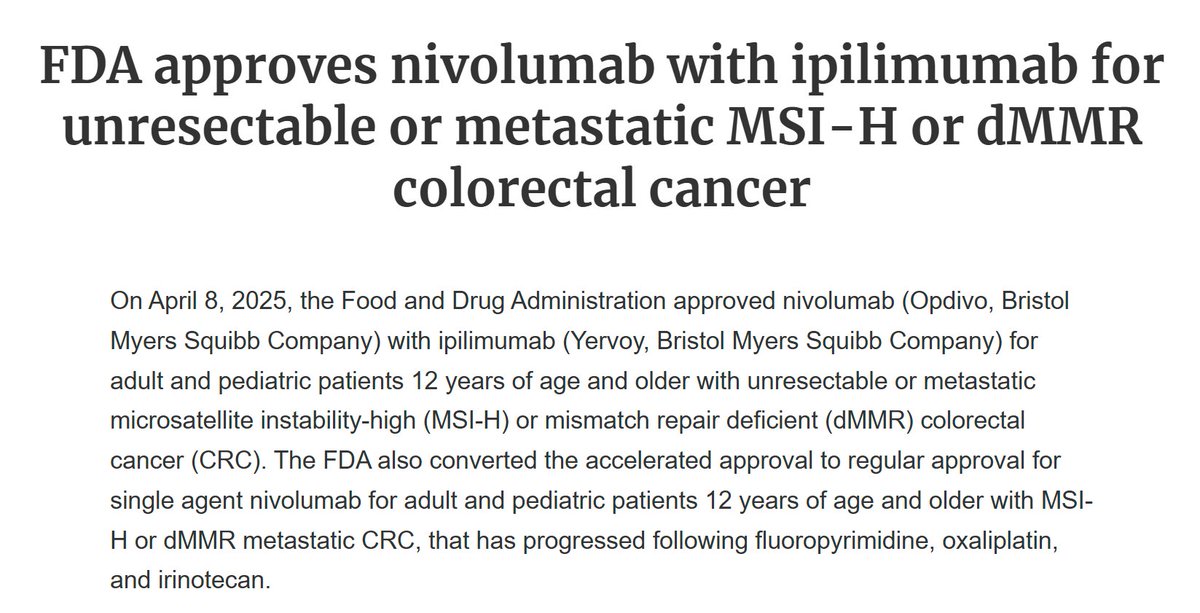
The #FDA has approved #nivolumab plus #ipilimumab for unresectable or metastatic microsatellite instability–high (#MSI-H) or mismatch repair deficient (#dMMR) colorectal cancer (#CRC). Learn more about the #approval and the #CHECKMATE8HW trial here:
0
0
0
Revolutionizing Treatment for MSI-H/dMMR Metastatic Colorectal Cancer: The Promising Results of the CheckMate 8HW Trial. #Immunotherapy #ColorectalCancer #OncologyResearch #ClinicalTrials #CheckMate8HW #GICancer #ASCO
0
0
0
《学会速報》.【大腸がん:一次治療】遠隔転移を有するMSI-H/dMMR大腸がんと診断された人が初めての治療を考える場合、「オプジーボ+ヤーボイ」治療を選択することで、「化学療法」を選択した場合を上回る2年無増悪生存率が期待できる。〔ASCO #GI24〕.
1
15
73
BMS Receives the US FDA’s Approval for Opdivo + Yervoy as a 1L Treatment of MSI-H/dMMR Metastatic Colorectal Cancer (mCRC).#bms #opdivo #yervoy #msihdmmrmcrc #pdufa #piii #checkmate8hw #regulatory #pharmashots
0
0
0
Happened this week: @FDA approves Nivo/Ipi vs. Nivo alone for #MSIhigh or #dMMR met #colorectal #cancer #Checkmate8HW .☑️Median PFS was NR in the combo arm vs. 39.3M (HR = 0.62 [95% CI: 0.48, 0.81] .☑️ORR = 71% vs. 58% (p-value 0.0011).@bmsnews #CancerResearch #immunotherapy
0
4
17