The Cesare Lab
@TheCesareLab
Followers
2K
Following
232
Media
191
Statuses
831
We study the mechanisms that maintain genome integrity in human cells and can now be found on Blue Sky: @thecesarelab.bsky.social
Sydney, Australia
Joined August 2013
Hello friends - we are now on Blue Sky: @thecesarelab.bsky.social We will continue to post here for the immediate future but expect a complete migration to Blue Sky in coming months. Please do reach out and connect with us there.
0
0
1
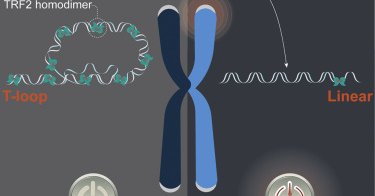
26/Finally, the data are consistent with t-loops suppressing ATM activation by the chromosome end. We show for the first time that t-loops are opened in a shelterin-dependent manner to signal cellular stress, suggestive of a broader role for these structures in cell biology.
0
0
0
25/We predict this represents non-canonical telomere-dependent tumour suppression activity outside the canonical ageing-dependent proliferative barriers imposed by telomere erosion. Also, we now know the protective functions of the TRF2 basic domain are mitosis specific.
1
0
0
24/What does this mean? We now understand the pathway of MAD-telomere deprotection that contributes to 1) cell death during mitotic arrest, and 2) p53-dependent cell cycle arrest when cells escape an elongated mitosis.
1
0
0
23/Live imaging from both labs revealed that TRF2 mutants which suppress MAD-telomere deprotection reduce mitotic arrest driven lethality, and TRF2 mutants that promote MAD-telomere deprotection, enhanced mitotic death and expedited time to mitotic lethality.
1
0
0
22/Of note, telomeres do not rapidly truncate in MAD-telomere deprotection like they do with TRF2 basic domain deletion. Modification of the TRF2 basic domain thus enables BTR-dependent t-loop opening while preventing t-loop junction cleavage, consistent with a regulated pathway.
1
0
1
21/Diana’s molecular biology was consistent with all BTR components, and BTR formation, being requisite for MAD-telomere deprotection following TRF2 modification. This suggested that t-loops junctions are in a dHJ onfiguration during mitotic arrest before resolution by BTR.
1
0
0
20/How does BLM fit into the story? BLM does many things, including double Holliday Junction (dHJ) dissolution via BTR. T-loops are typically drawn with strand-invasion promoting a displacement loop. However, this is conjecture, and the t-loops junction identify is unknown.
1
0
0
19/Ronnie's Airyscan imaging of t-loops following in situ trioxsalen cross-linking and chromatin spreading suggested TRF2 basic domain phosphorylation promoted the transition from looped to linear telomeres during mitotic arrest, coinciding with the telomere DDR activation.
1
0
0
18/Critically, both labs showed all TRF2 S62 and/or S65 phospo-null or -mimetic mutants retained interphase telomere protection, indicating these residues function specifically in mitotic telomere protection. Diana also showed that TRF1 and TRF2 mutants localize to telomeres.
1
0
0
17/Sam created a phosphospecific antibody against pTRF2-S65 and his biochemistry confirmed this residue was phosphorylated specifically during mitotic arrest in an AURKB-dependent fashion.
1
0
0
16/Within TRF2, we identified multiple AURKB consensus sites in the basic domain; the portion of TRF2 expected to protect t-loop junctions. Diana and Ronnie’s molecular biology was consistent with TRF2-S62 and S65 phosphorylation being requisite for MAD-telomere deprotection.
1
0
0
15/Survivin binding to pTRF1 suggested that CPC retention at mitotically arrested telomeres may promote AURKB modification of other shelterin factors. Because TRF2 regulates somatic t-loops, we investigated potential AURKB-dependent TRF2 phosphorylation.
1
0
0
14/Sam used phospho-peptides, mass spec, and biochemistry to identify that the CPC component Survivin bound to phosphorylated pTRF1. Diana’s molecular biology and live imaging confirmed that the CPC factors INCEMP and Survivin are requied for MAD-telomere deprotection.
1
0
0
13/Molecular biology by Diana suggested that TRF1 S354 and T358 phosphorylation were required for MAD-telomere deprotection. Sam created a phospho-specific antibody against pTRF1-T358 and found this residue was phosphorylated specifically during mitotic arrest by AURKB.
1
0
0
12/In silico analysis found AURKB consensus sites in the TRF1 BLM binding motif. To probe these, Diana depleted TRF1 and complemented with mutant alleles. To our surprise, depleting TRF1 suppressed MAD-telomere deprotection, indicating this is a shelterin dependent phenomenon!
1
0
0
11/To do so, Sam developed an unbiased and time resolved TRF1-APEX2 interactomics pipeline. This revealed that telomeres in mitotically arrested cells interacted with the CPC and BLM helicase. We were excited to find that BLM depletion suppressed MAD-telomere deprotection.
1
0
0
10/ We found that telomeres transition from looped to linear during mitotic arrest in an AURKB-dependent manner, corresponding with ATM-dependent telomere DDR. https://t.co/O0UhLBPYSa Telomeres thus change structure during mitotic stress and we wanted to know how his occurred.
cell.com
Van Ly et al. identify that telomere loops (t-loops) function as conformational switches that regulate ATM activity at chromosome ends. They find ATM activity is suppressed when telomeres adopt a...
1
0
0
9/Telomeres can adopt telomere loops (t-loops) where the distal chromosome end strand-invades the duplex telomeric DNA in cis. We and others suggested t-loops suppresses DDR activation by sequestering the chromosome terminus (an ATM substrate) within the telomeric DNA.
1
0
0
8/ MAD-telomere deprotection does require mitotic Aurora B kinase (AURKB) activity. It promotes p53 activation and a minority of death events during lethal mitotic arrest. This suggests MAD-telomere deprotection is a regulated signal that alerts cells to mitotic stress.
1
0
0
7/MAD-telomere deprotection is non-canonical (i.e. telomere length and ageing-independent). It is also agnostic to telomerase activity and does not damage the telomeres nor promote telomere fusions. MAD-telomere deprotection is also reversed in G1 if cells escape mitotic arrest.
1
0
0